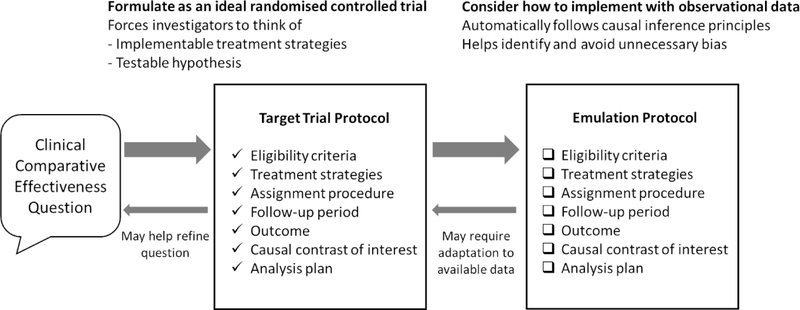
Figure 1.
The target trial protocol is used to guide observational comparative effectiveness research design. This idealized protocol may need to be reformulated once limitations of the data are realised. Divergence of the implemented observational study (emulation) from the target trial protocol should be addressed by sensitivity analyses or transparent reporting of limitations.