Figure 3.
Expanded teratoma-derived skeletal myogenic progenitors engraft and form new muscle fibers
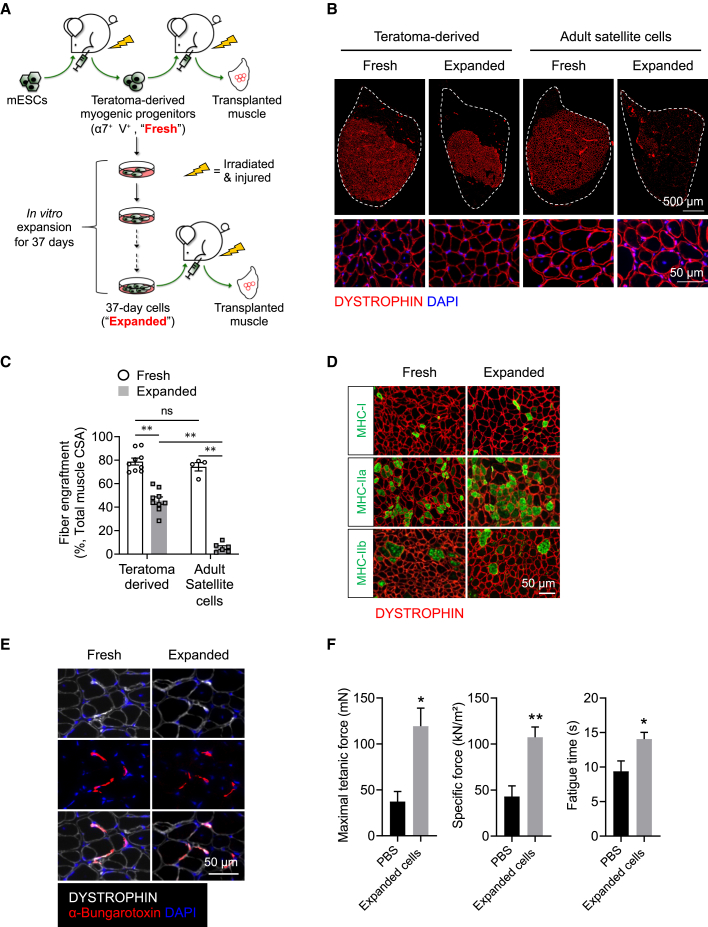
(A) Schematic of evaluation of the engraftability of freshly sorted and expanded teratoma-derived skeletal myogenic progenitors.
(B) Freshly sorted (far left) and expanded (middle left) teratoma-derived skeletal myogenic progenitors engrafted and formed DYSTOPHIN+ fibers 4 months post-transplant. In contrast, freshly sorted adult satellite cells (middle right) engrafted, but their expanded counterparts (far right) did not. The whole TA muscle is outlined (top, scale bar represents 500 μm), and magnified images are shown (bottom, scale bar represents 50 μm). Representative images from four to nine biological replicates.
(C) Quantification of fiber engraftment (DYSTROPHIN+ fibers) in transplanted TA muscles (n = 4–9 biological replicates). Data are shown as the mean ± SEM. ∗∗p < 0.01; ns, not significant.
(D) Newly formed DYSTROPHIN+ muscle fibers derived from freshly sorted and expanded cells consisted of slow-twitch (MHC-I) and fast-twitch (MHC-IIa and MHC-IIb) fibers (representative images from six biological replicates). Scale bar represents 50 μm.
(E) Potential presence of neuromuscular junctions as revealed by close proximity of α-bungarotoxin staining to newly formed DYSTROPHIN+ fibers derived from freshly sorted and expanded cells (representative images from three biological replicates). Scale bar represents 50 μm.
(F) In situ physiological assessment revealed functional improvement 4 months after transplantation of expanded teratoma-derived skeletal myogenic progenitors (n = 5–9 biological replicates). ∗p < 0.05, ∗∗p < 0.01 versus PBS (vehicle). ESCs, embryonic stem cells; α7, α7-integrin; V, VCAM-1; CSA, cross-sectional area.
See also Figures S2–S4.