Figure 2.
Reporter cell line reflects metabolic shift observed in maturing CMs
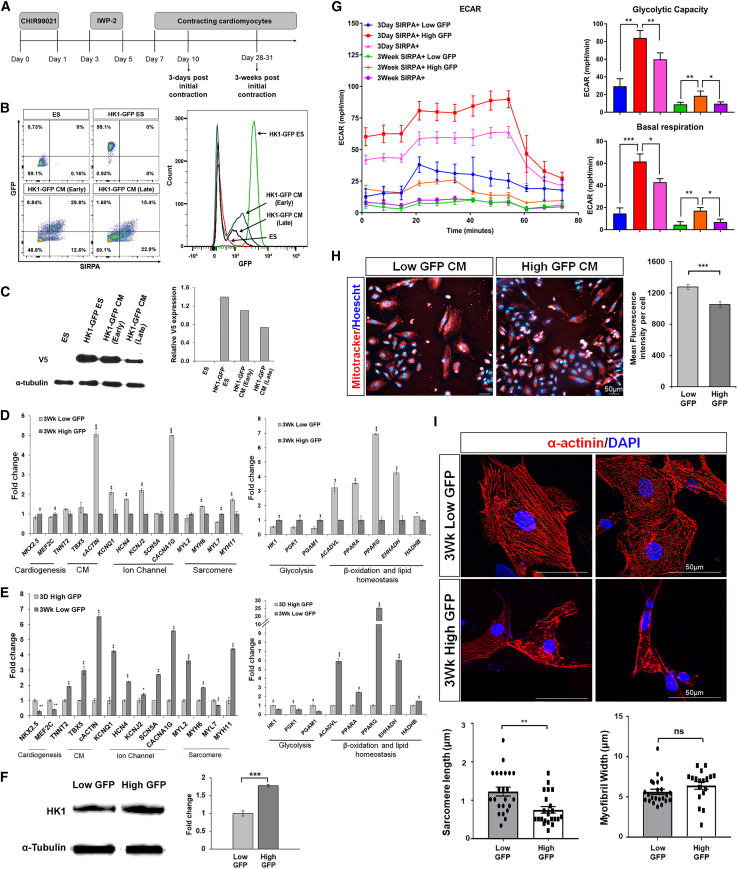
(A) Schematic diagram illustrating the differentiation protocol adopted to generate CMs from human embryonic stem cells. The differentiated CMs were analyzed at 3 days and 3 weeks post-initial contraction.
(B) Representative flow cytometry analysis of SIRPA+ CMs derived from H7 HK1-GFP reporter. Wild-type ESCs and HK1-GFP ESCs were used as a negative control. Percentages of SIRPA+/low- and high-GFP-expressing cells were compared between wild-type ESCs, HK1-GFP ESCs, 3-day post-initial contracting HK1-GFP CMs (early) and 3-week post-initial contracting HK1-GFP CMs (late). Representative histogram showing shifts in GFP expression as H7 HK1-GFP-derived cells differentiate to CMs. The experiments were repeated three times.
(C) Immunoblot showing decreasing V5 expression as H7 HK1-GFP reporter cells differentiate into early (3 days) and late (3 weeks) CMs. Graphical quantification of HK1-V5 protein expression as H7 HK1-GFP reporter cells differentiate into fetal and matured CMs. The experiments were repeated three times.
(D) Quantitative PCR analysis illustrated increased mRNA transcript expression of CMs, ion channel, sarcomere, β-oxidation, and lipid homeostasis genes, with decreased expression of cardiogenesis and glycolysis genes in 3-week post-initial contraction (expressing low GFP) compared with 3-week post-initial contraction (expressing high GFP) CMs. Data are represented as fold-change normalized to control samples, while expression of each gene was normalized to β-actin.
(E) Quantitative PCR analysis illustrated increased mRNA transcript expression of CMs, ion channel, sarcomere, β-oxidation, and lipid homeostasis genes, with decreased expression of cardiogenesis and glycolysis genes in 3-week post-initial contraction (expressing low GFP) compared with 3-day post-initial contraction (expressing high GFP) CMs. Data are represented as fold-change normalized to control samples, while expression of each gene was normalized to β-actin. For (D) and (E), data are presented as mean ± SD, n = 3 independently differentiated groups. Data information: statistical analysis was performed using Student’s two-tailed t test. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
(F) Western blot and densitometric analysis shows higher HK1 protein expression in 3-week high-GFP-expressing CM compared to 3-week low-GFP-expressing CM. Data are presented as mean ± SD, n = 3 independently differentiated groups. Data information: Statistical analysis was performed using students two-tailed t test. ∗∗∗p < 0.001.
(G) ECAR measurements using glycolysis stress assay was performed on 3-day and 3-week post-initial contracting CMs. Measurement of basal glycolysis and glycolytic capacity of (1) those expressing SIRPA+/low GFP, (2) SIRPA+/high GFP, and (3) SIRPA+ population only. Data represent mean ± SD, n = 3 independently differentiated groups.
(H) Immunostaining of low-/high-GFP-expressing CMs with MitoTracker (red) that stains mitochondria in live cells with Hoescht (blue). Mitochondrial content was measured based on mean fluorescence intensity per cell. Data represent mean ± SEM, n = 3 independently differentiated groups. Scale bars, 50 μm.
(I) Immunostaining and graphical quantification of sarcomere length (μm) (n = 40, p = 0.0002) and myofibril width (μm) (n = 40, p = 0.1664) in low-/high-GFP-expressing CMs stained with α-actinin (red). Nuclei were stained in blue with DAPI. Data represent mean ± SD, n = 25 cells from three independently differentiated groups. Scale bars, 50 μm. Statistical analysis was performed using Student’s two-tailed t test. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.