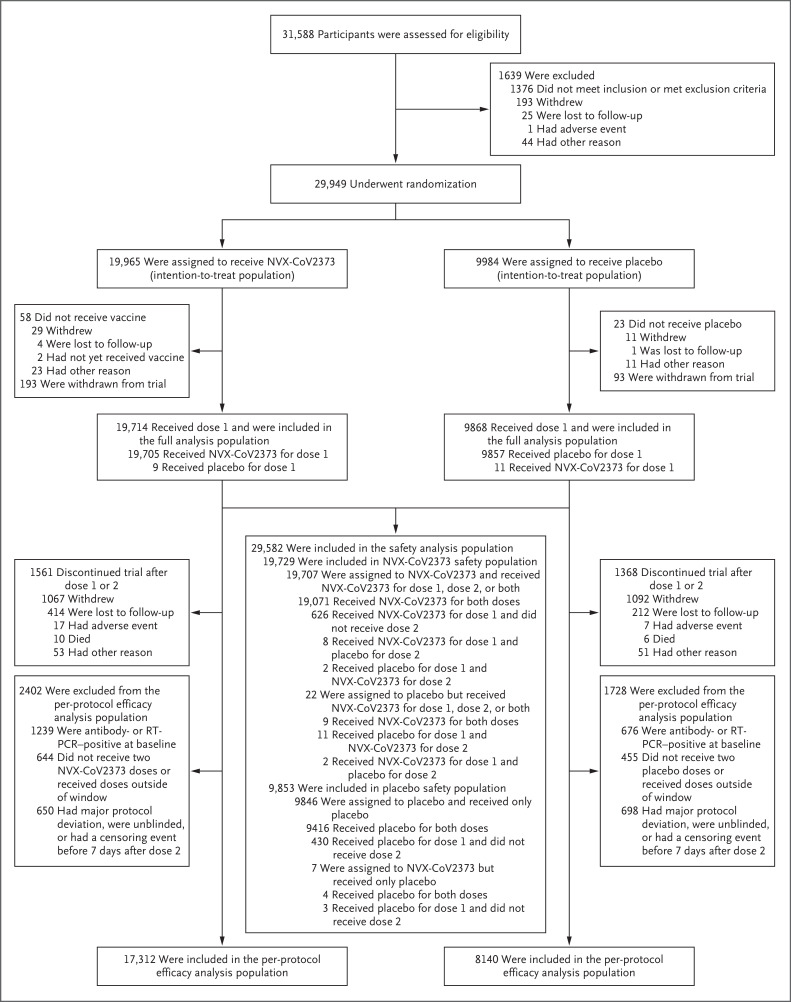
Figure 1. Screening, Randomization, and Follow-up.
The full analysis population included all participants who underwent randomization and received at least one dose of vaccine or placebo, regardless of protocol violations or missing data; participants in the full analysis population are included in the analysis according to the group to which they were randomly assigned. The safety analysis population included all participants who received at least one dose of vaccine or placebo. The per-protocol efficacy analysis population included all participants who underwent randomization and received both doses as assigned, were seronegative for anti–severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) nucleoprotein and had a SARS-CoV-2 RNA reverse-transcriptase–polymerase-chain-reaction (RT-PCR)–negative nasal swab at baseline, and did not have a censoring event at any time before 7 days after the second injection. Data from participants at two sites (193 assigned to receive NVX-CoV2373 and 93 assigned to receive placebo) were excluded from analyses because of Good Clinical Practice quality concerns. The window for the first dose of vaccine or placebo was December 20, 2020, to February 18, 2021.