Figure 1. NMDA receptor (NMDAR) signaling.
(A) Features of glutamatergic synapses. Vesicular release of glutamate (black dots) is triggered by Ca2+ influx through voltage-gated calcium channels (VGCCs) at the active zone. Glutamate, along with glycine or D-serine (gray dots), activate AMPAR and NMDAR on the postsynaptic membrane. AMPAR are anchored at the postsynaptic density by PSD-95 via auxiliary subunits (gray rectangle). NMDARs are clustered at the PSD by a direct interaction with PSD-95 (Kornau et al., 1995).
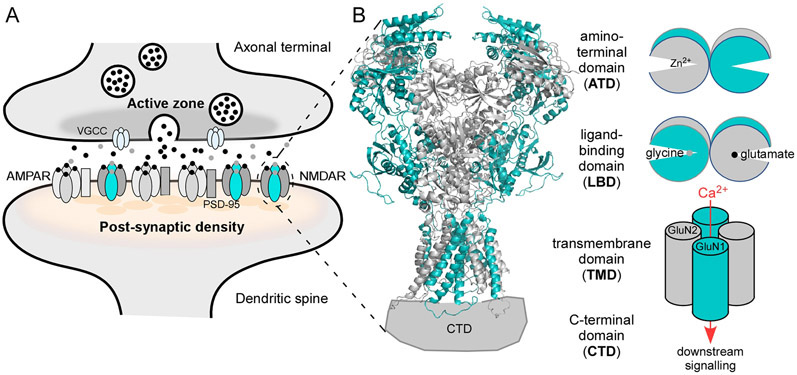
(B) Left, NMDAR topology. GluN1 is teal; GluN2 is gray. The tetrameric complex is composed of four highly modular domains: the extracellularly located amino-terminal (ATD) and ligand-binding (LBD) domains; the membrane-spanning transmembrane domain (TMD) forming the ion channel; and the intracellular C-terminal domain (CTD), which is not resolved in any iGluR structures. Model structure of GluN1/GluN2B (4TLM) (Amin et al., 2017, Amin et al., 2018).
(B) Right, In addition to charge transfer, NMDAR mediate a Ca2+ component of excitatory neurotransmission. The clam-shell like LBD and ATD regulate ion channel activity. Glycine (GluN1) and glutamate (GluN2) binding to the LBD, which induces clam-shell closure, directly leads to ion channel opening (Armstrong and Gouaux, 2000, Kazi et al., 2014); clam-shell closure of the ATD by agents like Zn2+ act as a negative allosteric modulator (Romero-Hernandez et al., 2016).