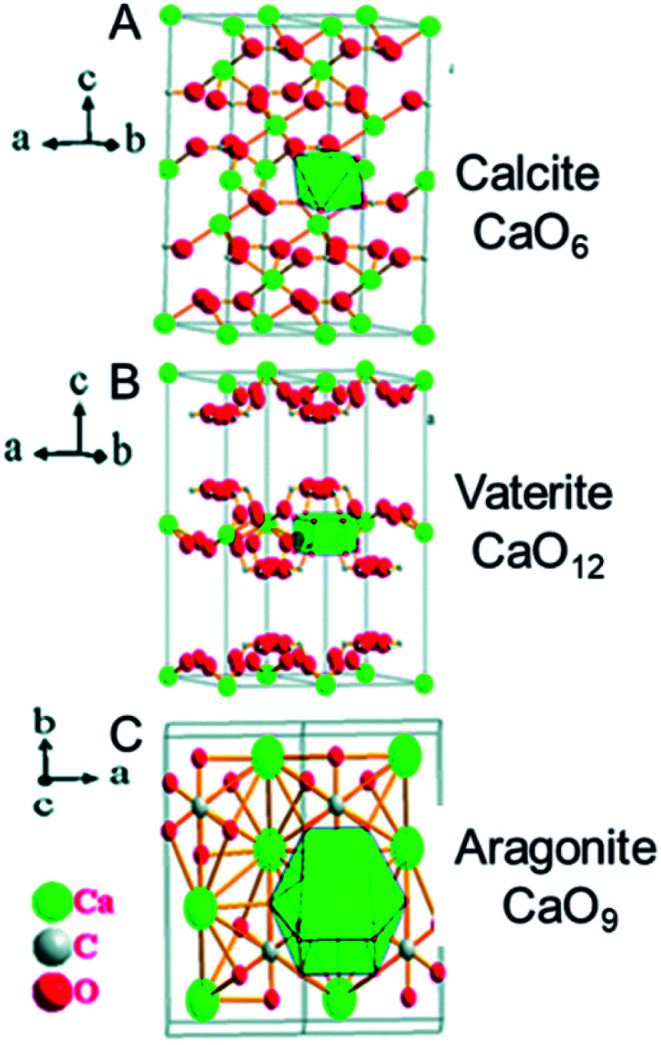
Fig. 6. X-ray crystal structures of calcium carbonate, CaCO3. (A) Calcite, (B) vaterite, and (C) aragonite. The coordination number of Ca2+ increases from 6 in calcite, to 9 in aragonite and 12 in vaterite (indicated on the right), which is accompanied by an increase in ligand repulsion, and a decrease in stability. The most stable form, calcite, is found in the human ear. Reproduced from ref. 35 with permission from the Royal Society of Chemistry, copyright 2017.35.