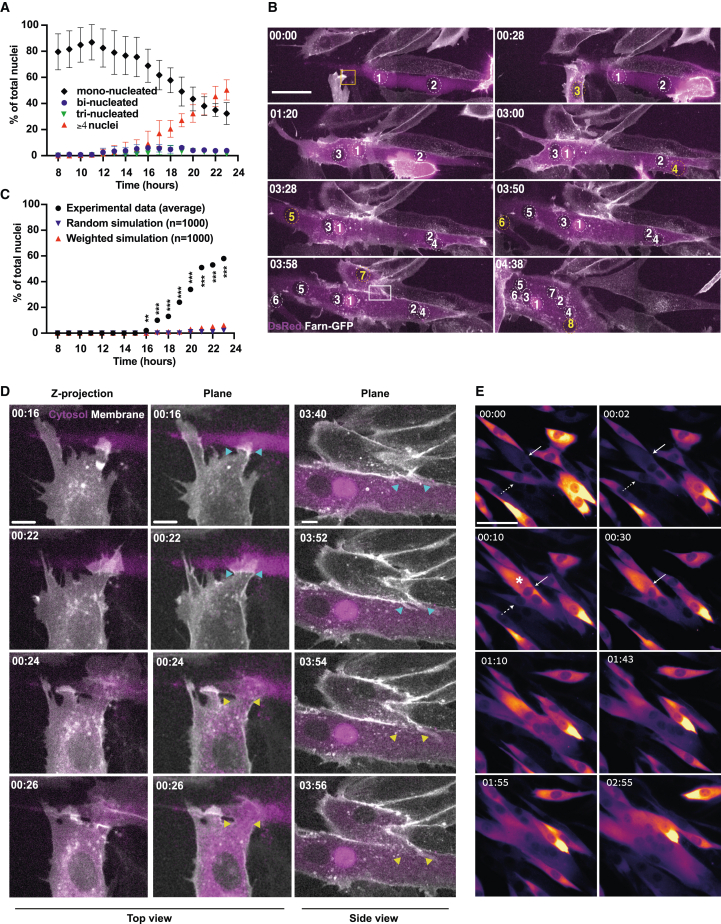
Figure 2.
Myotubes grow through recruitment of mononucleated myoblasts at a fusogenic synapse
(A) Hourly fusion index showing the distribution of mono-, bi-, tri-, and multinucleated (n 4) cells. Total number of nuclei assayed, n = 13,044.
(B) Representative frames from time-lapse microscopy of an individual growing myotube (Video S2). At time 0 a binucleated myotube labeled with a cytoplasmic DsRed (purple) is approached by a mononucleated myoblast (yellow square) expressing a membrane-targeted GFP (white). When the cells fuse, cytoplasmic and membrane mixing become apparent (t = 00:28). Scale bar: 50 μm. Yellow and white squares mark the fusion events shown in (D).
(C) Experimental data compared with simulated data in two stochastic fusion scenarios: equal probability of cells to fuse irrespective of their number of nuclei (4 nuclei), and weighted probability, which considered the possibility that the probability of a cell to add nuclei was proportional to the number of nuclei within it (see STAR Methods for full details).
(D) Two examples of “fusogenic synapses” from the expanding fiber in (B) (time: hh:mm). Scale bar: 10 μm. Left column: Z-projection of the confocal stack. A protrusion extending from the myoblast to the myotube where fusion eventually occurs as can be seen by the simultaneous diffusion of the cytoplasmic marker into the myoblast and the disappearance of the membrane marker from the protrusion between the two fusing cells (Video S4). Middle and right columns: focal planes from two events where the fusion pore can be seen expanding. Cyan and yellow arrows point to the fusogenic synapses before and after fusion.
(E) Representative frames acquired of GCaMP6S Ca2+ reporter fluorescence in a growing myotube undergoing expansion via fusion (Video S5). Arrows indicate a myoblast and a small myotube before fusion and the initiation of fiber growth. Dashed arrow indicates a myoblast prior to and during fusion. Asterisk indicates burst in GCaMP6S fluorescence. Scale bar: 50 μm. Time in (B), (D), and (E) (hh:mm).