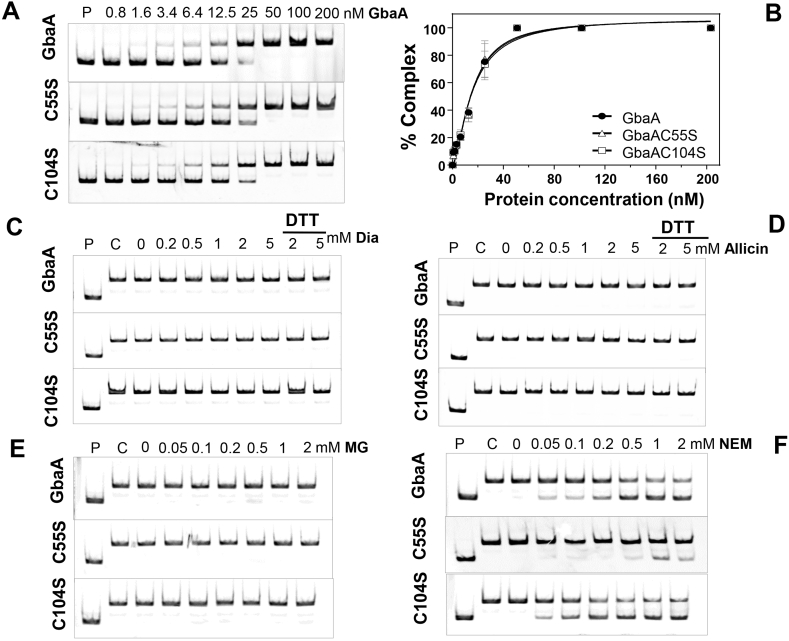
Fig. 5.
The DNA binding activity of GbaA and the Cys mutant proteins is not inhibited under disulfide stress (diamide, allicin) and MG, but partially affected by NEM in vitro. (A) EMSAs were used to analyze the DNA binding activity of increasing concentrations of GbaA, GbaAC55S and GbaAC104S proteins to the 150 bp gbaA promoter probe. (B) The percentage of the GbaA-DNA complex formation was determined according to the band intensities of five biological replicates of the EMSAs in A) and quantified using Image J 1.48v. Dissociation constants (KD) were calculated as 15.24 nM, 15.24 nM and 15.79 nM for GbaA, GbaAC55S and GbaAC104S mutant proteins, respectively using the Graph prism software version 6.01. (C-F) The DNA binding activity of GbaA, GbaAC55S and GbaAC104S proteins was not affected by diamide, allicin and MG (C–E), but partially inhibited with NEM (F).