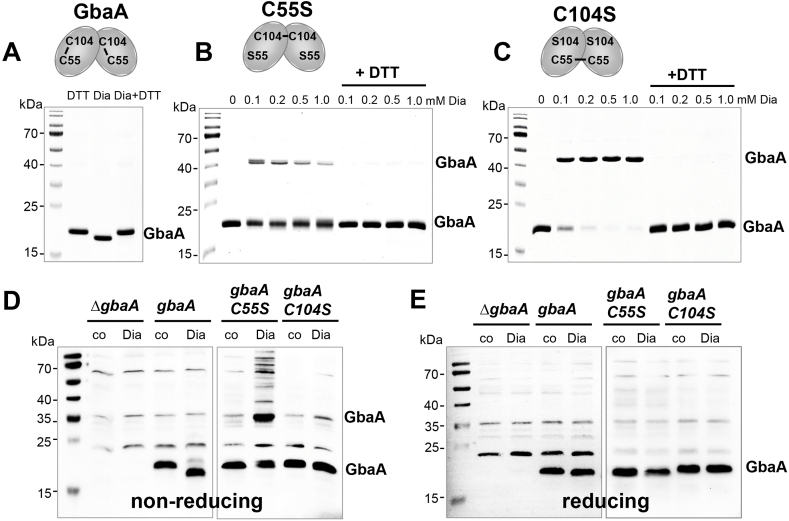
Fig. 6.
GbaA and the GbaA Cys mutants are oxidized to intra- and intermolecular disulfides by diamide in vitro and in vivo, respectively. (A–C) Purified GbaA was treated with 1 mM diamide (A), while the GbaAC55S (B) and GbaAC104S mutant proteins (C) were exposed to increasing concentrations of diamide for 15 min, followed by alkylation with 50 mM IAM for 30 min in the dark and separation by non-reducing SDS-PAGE. The non-reducing SDS-PAGE gels are stained with Coomassie Blue. To assess the reversibility, diamide-treated samples were reduced with 20 mM DTT for 15 min before alkylation and analysis by non-reducing SDS-PAGE. GbaA is oxidized to intramolecular C55-C104 disulfides by diamide as confirmed by MALDI-TOF MS (Fig. S7), while the C55S and C104S mutants form intermolecular disulfides as shown in the schematics above the gel images. (D, E) The S. aureus gbaA mutant and the gbaA complemented strain were treated with 5 mM diamide and the gbaAC55S and gbaAC104S complemented strains were exposed to 2 mM diamide for 30 min, alkylated with NEM and the protein extracts analyzed for thiol-oxidation of GbaA in vivo by non-reducing (D) and reducing (E) Western blot analysis with monoclonal anti-His6 tag antibodies. The protein loading controls are shown in Fig. S8.