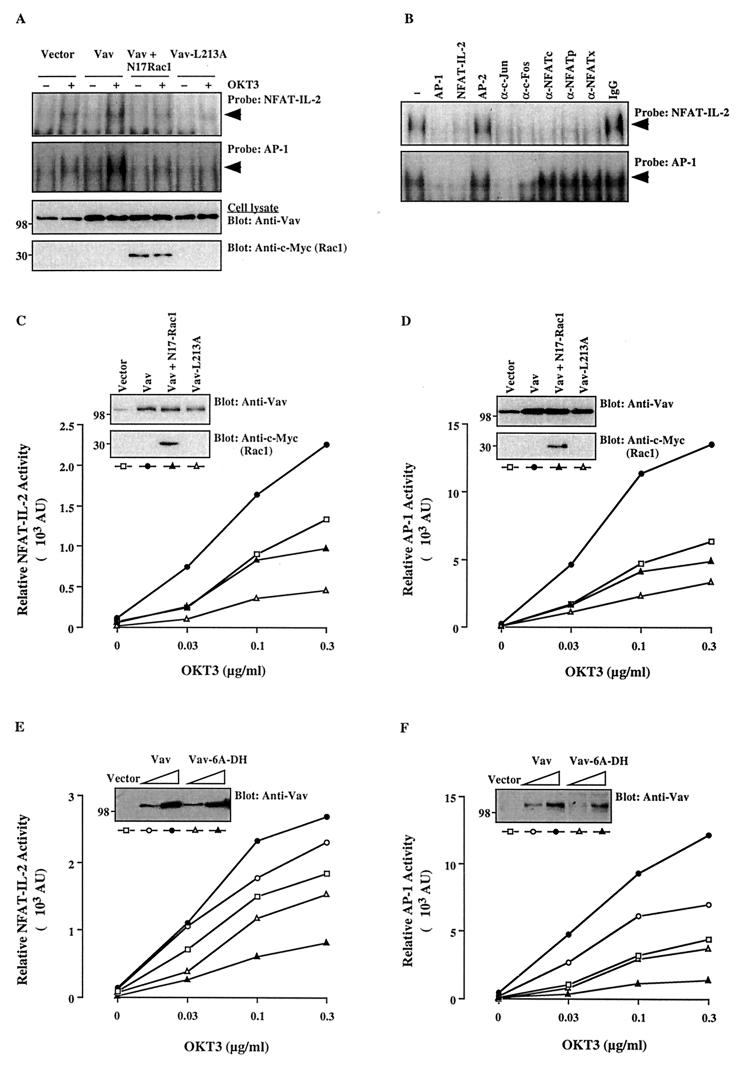
FIG. 1.
The Vav/Rac pathway plays a role in NFAT–IL-2 and AP-1 activation. (A) Jurkat-TAg cells were transfected with empty pEF vector (10 μg) or wild-type Vav, Vav-L213A, or Vav plus N17Rac1 (5 μg each). After 24 h, cells (107/ml) either were left unstimulated or were stimulated with cross-linked OKT3 (0.3 μg/ml). Nuclear extracts were analyzed by an EMSA using NFAT–IL-2 and AP-1 oligonucleotide probes. The membranes were subjected to autoradiography (top two panels), and total cellular extracts from the same cells were immunoblotted with anti-Vav or anti-c-Myc antibodies (bottom two panels). The results shown are representative of four separate experiments. Arrowheads indicate specific NFAT and AP-1 complexes. (B) A similar EMSA was performed using a nuclear extract from OKT3-stimulated, Vav-transfected cells in the absence (−) or presence of the indicated unlabeled competing oligonucleotides, antibodies, or control IgG. A similar inhibition pattern was observed with an extract from unstimulated, Vav-transfected cells (data not shown). (C to F) Cells were cotransfected with NFAT–IL-2–Luc (C and E) or AP-1–Luc (D and F) reporter plasmids plus the indicated combinations of Vav, Vav-L213A, Vav-6A-DH, and/or N17Rac1. After 24 h, the cells either were left unstimulated or were stimulated with the indicated concentrations of cross-linked OKT3. Luciferase and β-Gal activities in cell extracts were determined 8 h later. AU, arbitrary units. The standard deviation for triplicates was <10%, and each experiment was repeated four times, with similar results. Samples of the same lysates were analyzed for the expression of Vav and N17Rac1 by immunoblotting with anti-Vav and anti-c-Myc antibodies, respectively (insets). The positions of molecular weight standards (in thousands) are shown.