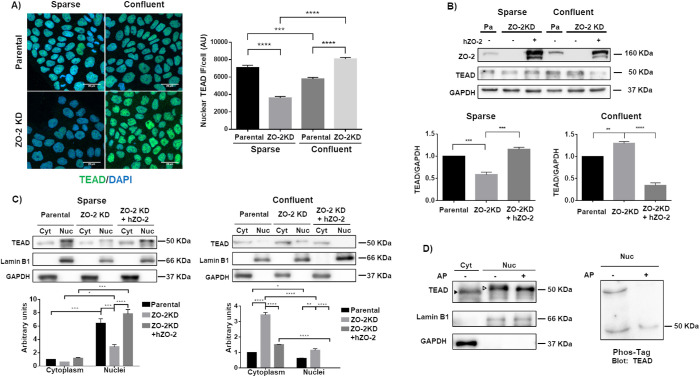
FIGURE 1:
ZO-2 facilitates the nuclear accumulation of TEAD. (A) In ZO-2 KD cells nuclear TEAD was diminished in sparse cultures, while cytoplasmic TEAD increased in confluent monolayers. Sparse and confluent cultures of parental and ZO-2 KD MDCK cells were processed for immunofluorescence with antibodies against TEAD. Left, representative images; right, quantitative analysis. The nuclei of 100 cells per condition derived from three independent experiments were quantitated. Statistical analysis done with Kruskal–Wallis test followed by Dunn’s multiple comparisons test. AU, arbitrary units. ***p < 0.001; ****p < 0.0001. (B) In ZO-2 KD cells, the amount of TEAD decreased in sparse cultures and increased in confluent cultures. Western blot of total cellular extracts derived from sparse and confluent cultures of parental and ZO-2 KD cells transfected or not with hZO-2. GADPH was employed as loading control. Top panel, representative image of three independent experiments; bottom panel, densitometric analysis. Statistics done with one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test, **p < 0.01; ***p < 0.001. (C) The lack of ZO-2 diminished the amount of TEAD at the nucleus of sparse cells and increased cytoplasmic TEAD in confluent monolayers. Western blot detection of TEAD in cytoplasmic (Cyt) and nuclear (Nuc) fractions derived from sparse and confluent cultures of parental and ZO-2 KD cells transfected or not with hZO-2. Lamin B1 and GAPDH were employed as markers of nuclear and cytosolic fractions, respectively. Top panels, representative images of three independent experiments; bottom panels, quantitative analysis of TEAD/lamin B1 in the nuclear fractions and of TEAD/GAPDH in the cytoplasmic fractions. Statistics done with two-way ANOVA followed by Tukey’s multiple comparisons test, *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001. (D) Nuclear TEAD has a higher molecular weight than cytoplasmic TEAD due to phosphorylation. Left panel, Western blot detection of TEAD in cytoplasmic (Cyt) and nuclear (Nuc) fractions treated or not with alkaline phosphatase (AP). Antibodies against lamin B1 and GAPDH were employed as markers of nuclear and cytoplasmic fractions, respectively. Representative image of two independent experiments. Filled arrowhead, 49 kDa band; empty arrowhead, 51 kDa band. Right panel, nuclear (Nuc) fractions treated or not with AP were run on an SDS–PAGE with acrylamide-pendant Phos-tag, and blotted with antibodies against TEAD.