Fig. 4.
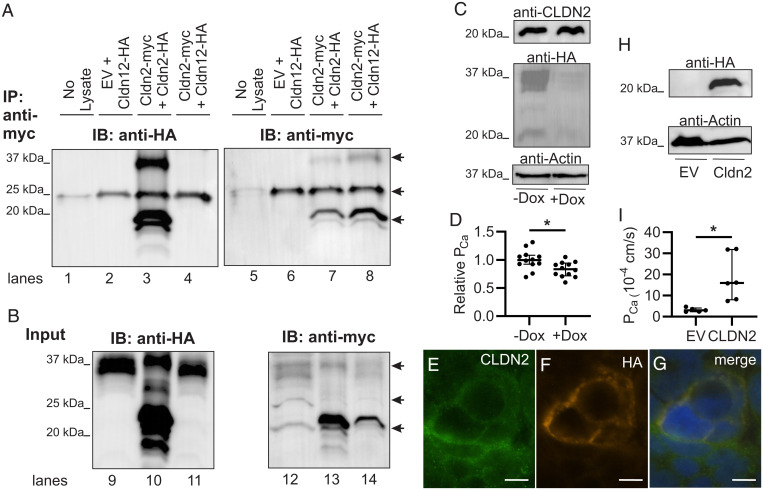
Claudin-2 and claudin-12 form separate Ca2+ permeable pores. (A) HEK293 cells transfected as indicated were lysed or the buffer without cell lysate as control (no lysate lane) and proteins immunoprecipitated (IP) with antibodies to myc before sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE). Proteins were electrotransferred and blotted with anti-HA antibody (lanes 1 through 4) and then stripped and reprobed with an anti-myc antibody (lanes 5 through 8). IB, immunoblot. (B) Protein from HEK293 cells above directly resolved on SDS-PAGE (Input) and blotted with anti-HA (lanes 9 through 11) and then anti-myc (lanes 12 through 14). Claudin dimers (37 kDa) and monomers (20 kDa) are highlighted by arrows. The arrow at 25 kDa illustrates IgG light chain in the IP samples as seen in the lane with no cell lysate added. EV, empty vector. Representative blot of three repeats is shown. (C) Caco-2 cells endogenously express CLDN2 and express HA-tagged CLDN12 via a tet-off system. (D) Caco-2 cells expressing CLDN2 and CLDN12 (−Dox) have greater Ca2+ permeability than cells expressing only CLDN2 (+Dox) (P = 0.014 and unpaired t test). (E–G) Immunofluorescence of Caco-2 cells for endogenous CLDN2 (green) and HA-tagged CLDN12 (orange) demonstrating colocalization of CLDN2 and CLDN12 in the same cell membrane. (H) Opossum kidney (OK) cells endogenously expressing CLDN12 and expressing either EV or CLDN2. (I) OK cells expressing CLDN2 and CLDN12 (“CLDN2” data) have greater Ca2+ permeability than cells expressing only CLDN12 (“EV” data) (P = 0.018, Welch’s t test). Dox, doxycycline. TER, pNa, pCl, and relative permeabilities are presented in SI Appendix, Tables S8 and S9. *P < 0.05.