FIGURE 6:
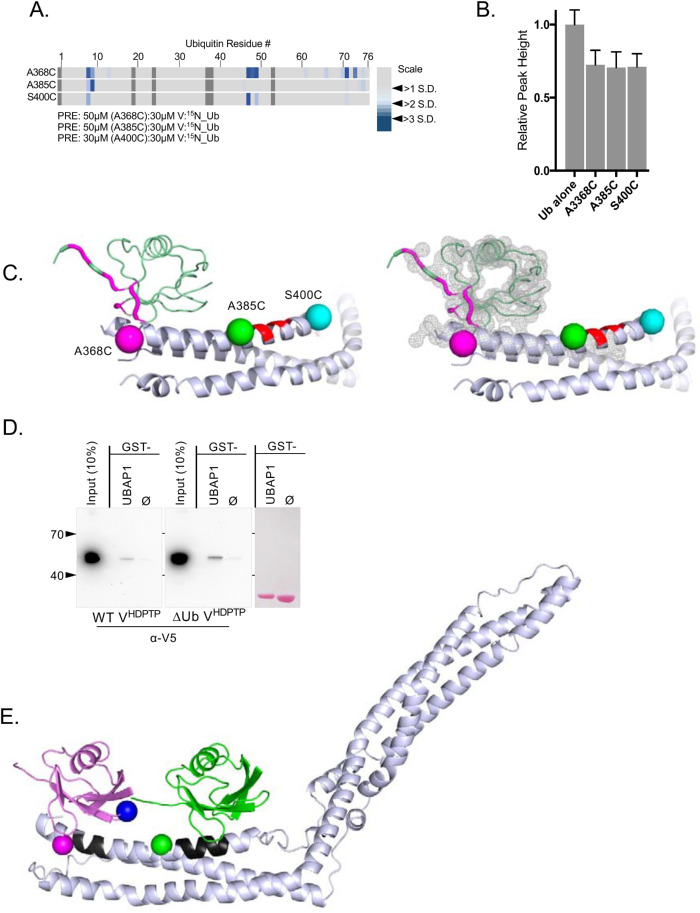
PRE mapping of Site1 Ub binding. (A) Paramagnetic relaxation effects on backbone amides of 15N-Ub using HD-PTP V domains containing single spin labels at the indicated positions (residues 368, 385, or 400) also containing mutations disrupting the Site2 Ub-binding site (E388A, D389A). The ratio of the V domain to 15N-Ub is shown. The largest paramagnetic enhancement effects, measured by the change in the ratio of peak intensity before and after reduction, are plotted by color indicating those that were 2–3 SDs from the mean change. (B) Relative average peak heights (±SD) of 30 µM 15N-Ub HSQC spectra alone or in the presence of 50 or 30 µM HD-PTP MTSL-labeled variant proteins in the presence of 2 mM ascorbate as described for A. (C) Positioning of Ub over Site1. Shown are positions of MTSL labeling used in A. The largest PRE effects on 15N Ub when bound to the V domain spin labeled at residue 368 were mapped onto the structure of Ub in magenta. Right, The fit of the HD-PTP:Ub model into the electron density map for the VBro1:Ub crystal structure (4JIO). (D) Pull-down experiments with WT or ∆Ub mutant HD-PTP V (Site1∆: L371A, Y372A, E374A; Site2∆: E388A, D389A, E392A) to assess binding to GST alone or GST fused to residues SNIKSLSFPKLDSDDSNQKT of UBAP1 that mediate binding to the distal C-terminal arm of the HD-PTP V domain. (E) Model of Ub bound to Site1 (black) and Site2 (black). Positions for PRE experiments at residue 368 (magenta) and 385 (green) of VHDPTP or position of K63 of Ub at Site1 (blue) are shown.