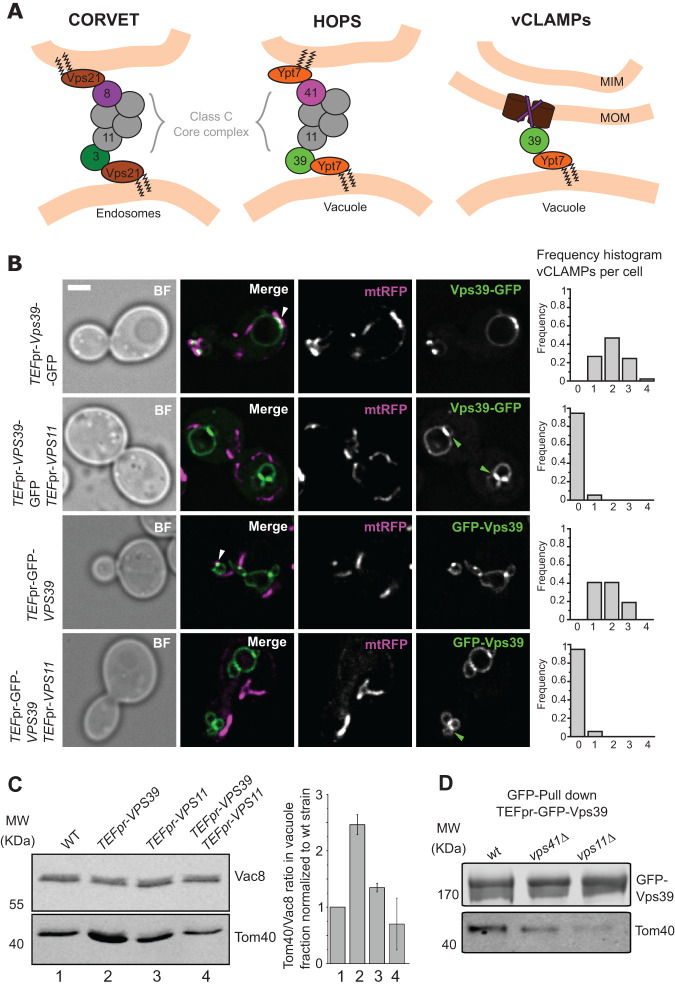
FIGURE 2:
HOPS and vCLAMPs compete for Vps39. (A) Diagram depicting the proteins that form the endolysosomal tethering complexes and the Vps39–vCLAMP tether. The Vps39 protein is present in both HOPS and vCLAMP, and the Class C-core complex, formed by Vps33, Vps16, Vps18, and Vps11, is part of both HOPS and CORVET. (B) Analysis of the effect of Vps11 overexpression on extended vCLAMP formation. Overexpressed Vps39 tagged with GFP at either the N- or C-terminus was imaged relative to mitochondrial targeted RFP (mtRFP). Where indicated, Vps11 was overexpressed under the control of TEF1 promoter. White arrowheads indicate overextended vCLAMPs, and the frequency of observation of these structures is represented in the frequency histogram to the right of the images. Green arrowheads indicate accumulations of Vps39 between two vacuoles. The scale bar represents 2 μm. (C) Examination of mitochondrial copurification in vacuole preparations. Vacuoles were purified from the indicated strains. Western blotting was used to evaluate the copurification of mitochondria by analyzing the levels of Tom40 (mitochondria marker) and Vac8 (vacuolar marker) in the purified vacuoles. The plot to the right displays the ratio Tom40/Vac8 normalized to WT, as mean ± SD of three independent experiments. (D) Coimmunoprecipitation of Vps39 and Tom40 is diminished in strains with defective HOPS. Vps39 was N-terminal GFP-tagged and overexpressed under control of TEF1pr in strains containing deletions in VPS41 of VPS11. Vps39 was immunoprecipitated with GFP-TRAP Sepharose beads, and eluates were analyzed by SDS–PAGE and Western blotting for levels of Vps39 and copurified Tom40.