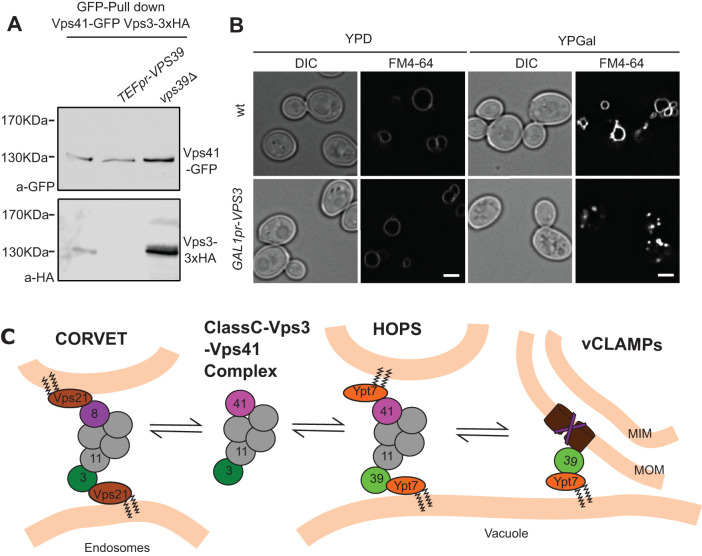
FIGURE 4:
The formation of different tethering complexes is determined by an equilibrium affected by the protein levels of the subunits. (A) Coimmunoprecipitation of Vps41 and Vps3 at different levels of Vps39. C-terminal GFP-tagged Vps41 was immunoprecipitated with a GFP-nanobody Sepharose affinity matrix from strains containing endogenous levels of Vps39, overexpressed Vps39 from the TEF1 promoter, or a deletion of VPS39. Eluates were analyzed by SDS–PAGE and Western blotting for levels of Vps41 and copurified Vps3–3xHA. (B) Vacuole morphology upon overexpression of Vps3. The morphology of vacuoles in cells overexpressing Vps3 from the strong GAL1 promoter was analyzed by FM4-64 staining of the vacuole membrane. The scale bar represents 2 μm. (C) Working model depicting the existence in vivo of an intermediate Class C–Vps3–Vps41 complex. Availability of the subunits shared with HOPS affect the levels of this complex, which could act as a buffering mechanism to allow the participation of Vps39 in the vCLAMP membrane contact site. For details see text.