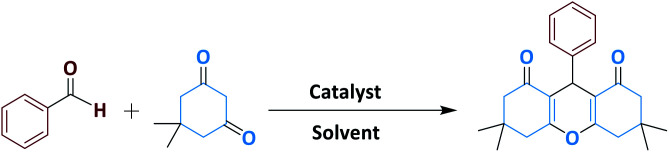
Optimization of reaction conditions for synthesis of 3,3,3,6,6-pentamethyl-9-phenyl-3,4,5,6,7,9-hexahydro -1H-3,5-xanthene-1,8(2H)-dione.
| |||||
|---|---|---|---|---|---|
| Entry | Solvent | Catalyst (mol%) | Temp. (°C) | Time (min) | Yielda (%) |
| 1 | EtOH : H2O | 0.5 | 45 | 15 | 90 |
| 2 | H2O | 0.5 | 45 | 20 | 85 |
| 3 | EtOH | 0.5 | 45 | 10 | 96 |
| 4 | MeOH | 0.5 | 45 | 10 | 85 |
| 5 | CH3CN | 0.5 | 45 | 60 | 70 |
| 6 | EtOAC | 0.5 | 45 | 75 | 65 |
| 7 | CHCl3 | 0.5 | 45 | 45 | 85 |
| 8 | Neat | 0.5 | 45 | 45 | 60 |
| 9 | EtOH | — | 45 | 10 | Trace |
| 10 | EtOH | 1 | 45 | 10 | 95 |
| 11 | EtOH | 1.5 | 45 | 10 | 90 |
| 12 | EtOH | 0.5 | — | 60 | 55 |
| 13 | EtOH | 0.5 | 55 | 10 | 90 |
| 14 | EtOH | 0.5 | 65 | 10 | 85 |
| 15 | EtOH | 0.5 | 45 | 20 | 96 |
| 16 | EtOH | 0.5 | 45 | 40 | 92 |
| 17 | EtOH | 0.5 | 45 | 60 | 85 |
| 18 | EtOH | 0.5b | 45 | 60 | 0 |
| 19 | EtOH | 0.5c | 45 | 60 | 0 |
| 20 | EtOH | 0.5d | 45 | 30 | 70 |
Reaction conditions: benzaldehyde (1 mmol) and dimedone (2 mmol). The catalyst was prepared by using 0.5 mol% of catalyst as explained in the Experimental section.
Reaction was performed in the presence of Fe3O4 as the catalyst.
Reaction was performed in the presence of Fe3O4@NFC as the catalyst.
Reaction was performed in the presence of Fe3O4@NFC@NNS-Mn(iii) as the catalyst.
