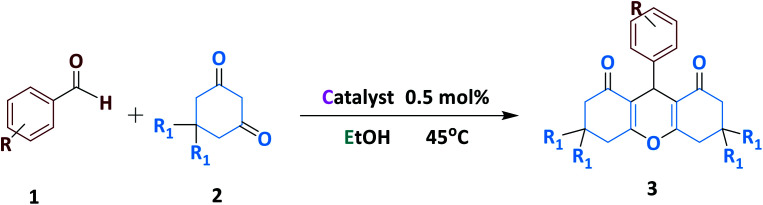
One-pot three-component synthesis of various xanthene derivatives catalysed by Fe3O4@NFC@NNSM-Mn(iii)a.
| |||||||
|---|---|---|---|---|---|---|---|
| Entry | Aldehyde | R 1 | Product | Time (min) | Yieldb (%) | MP (°C) | |
| Found | Reported | ||||||
| 1 (ref. 53) |
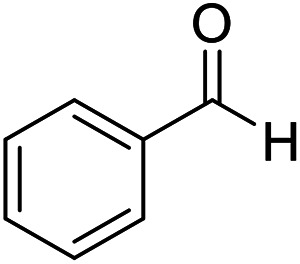
|
H |
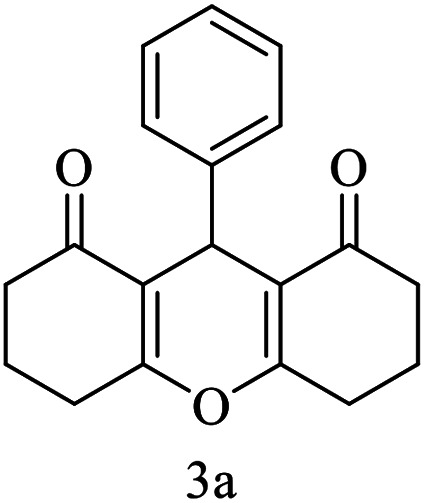
|
10 | 98 | 203–204 | 203–205 |
| 2 (ref. 53) |
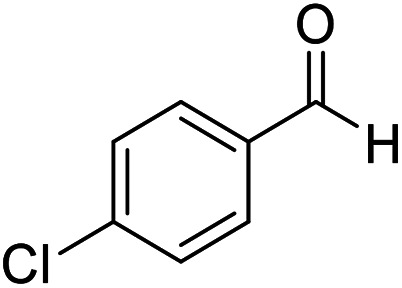
|
H |
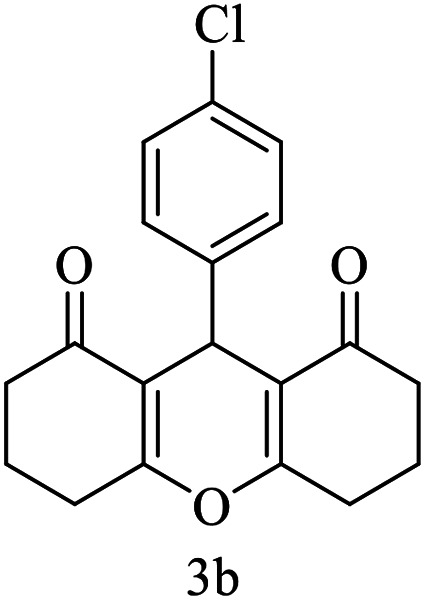
|
15 | 95 | 229–230 | 228–230 |
| 3 (ref. 54) |
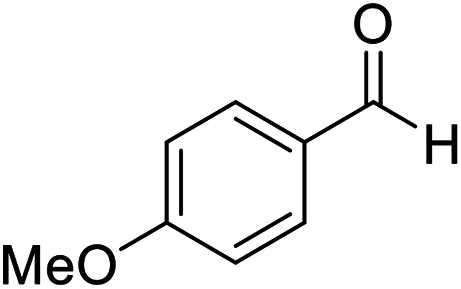
|
H |
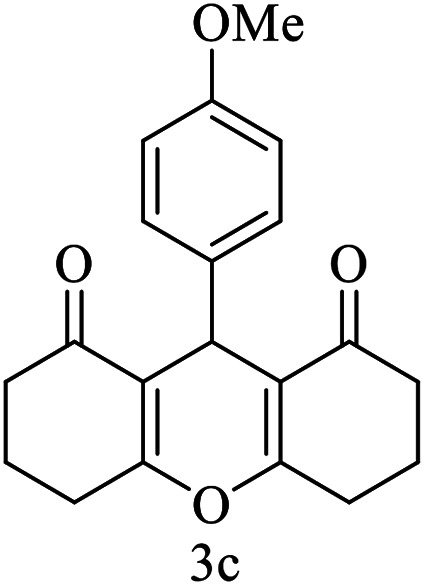
|
15 | 92 | 205–206 | 206–207 |
| 4 (ref. 53) |
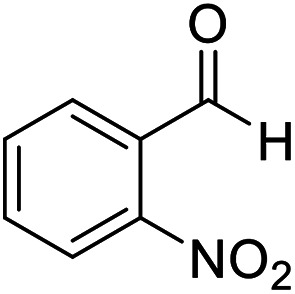
|
H |
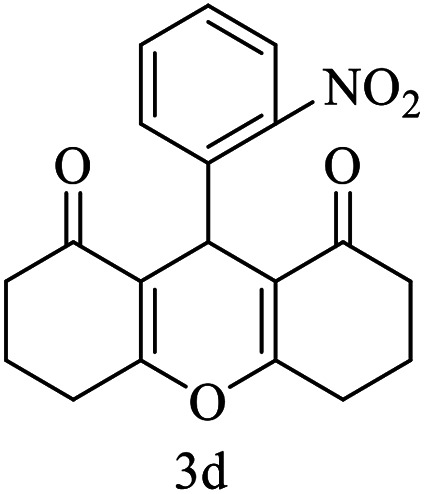
|
10 | 95 | 259–261 | 260–261 |
| 5 (ref. 55) |
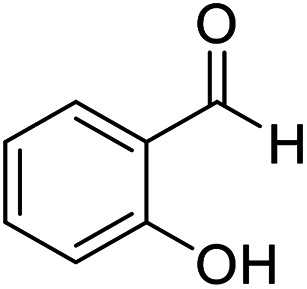
|
H |
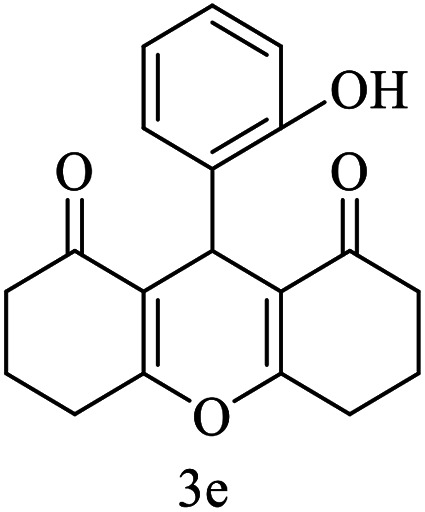
|
10 | 97 | 205–206 | 205–206 |
| 6 (ref. 56) |
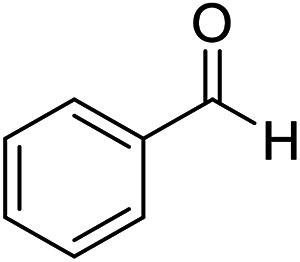
|
Me |
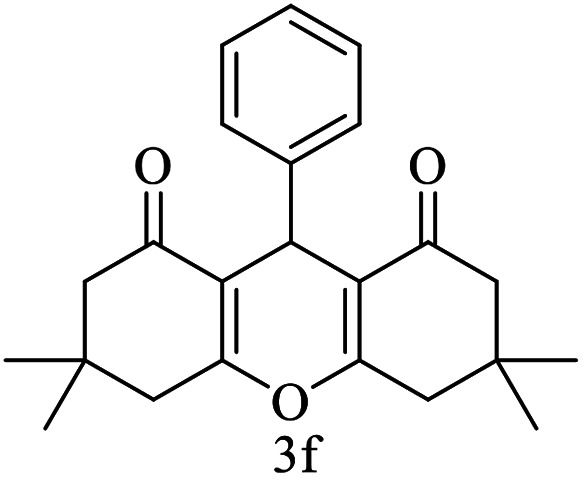
|
10 | 96 | 203–204 | 202–204 |
| 7 (ref. 57) |
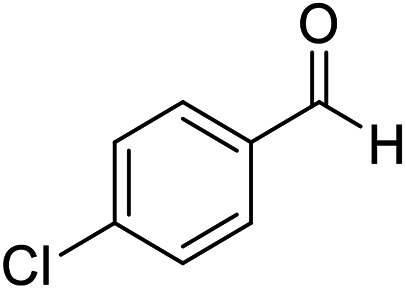
|
Me |
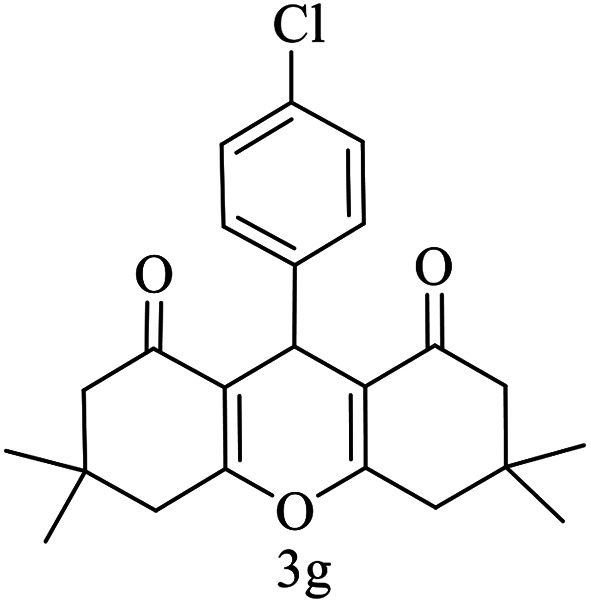
|
10 | 95 | 169–170 | 168–170 |
| 8 (ref. 58) |
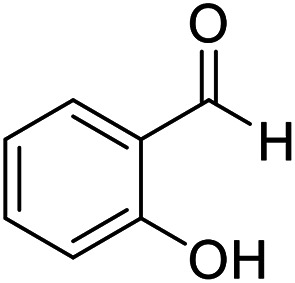
|
Me |
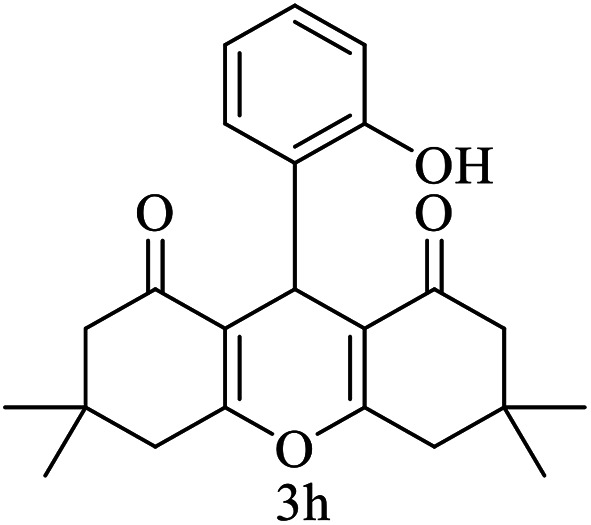
|
10 | 96 | 200–201 | 201–202 |
| 9 (ref. 59) |
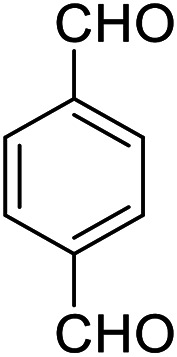
|
Me |
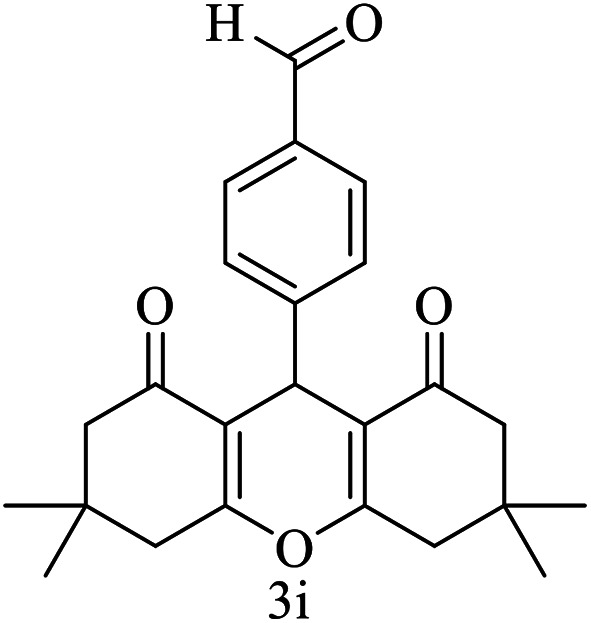
|
20 | 93 | 295–297 | >300 |
| 10 (ref. 60) |
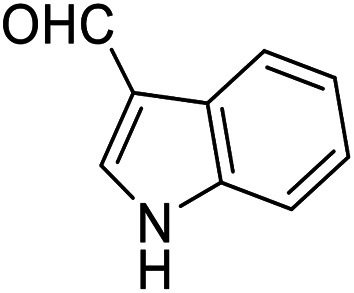
|
H |
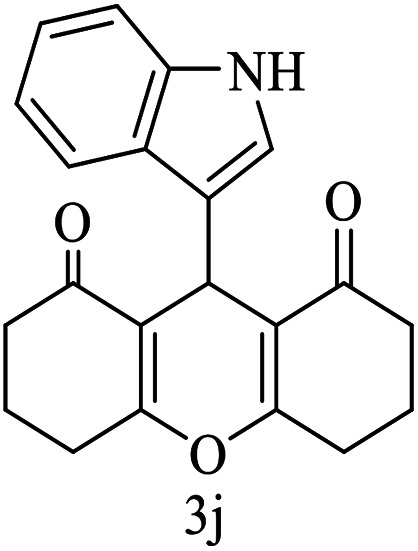
|
30 | 80 | 267–269 | 268–269 |
| 11 (ref. 61) |
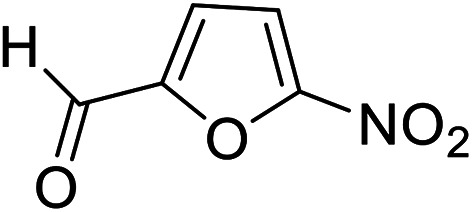
|
Me |
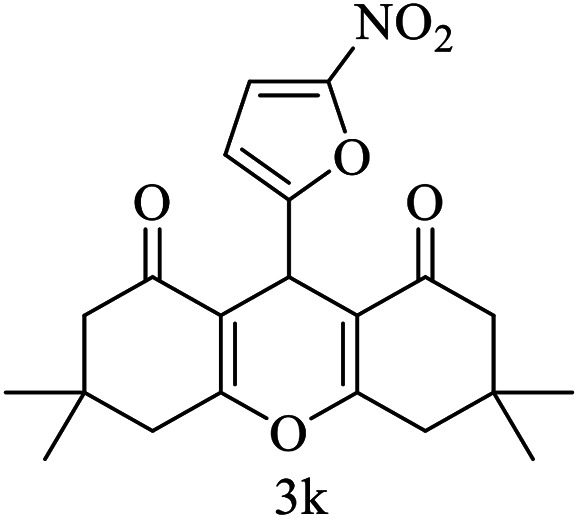
|
25 | 85 | 155–157 | — |
| 12 |
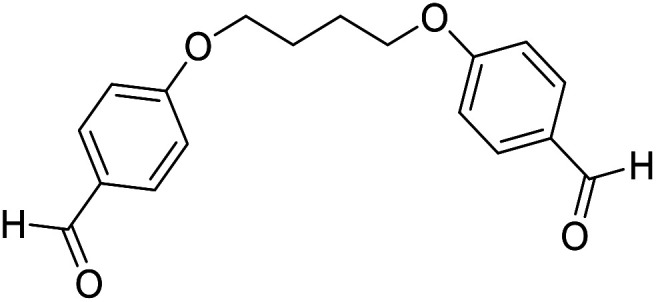
|
H |
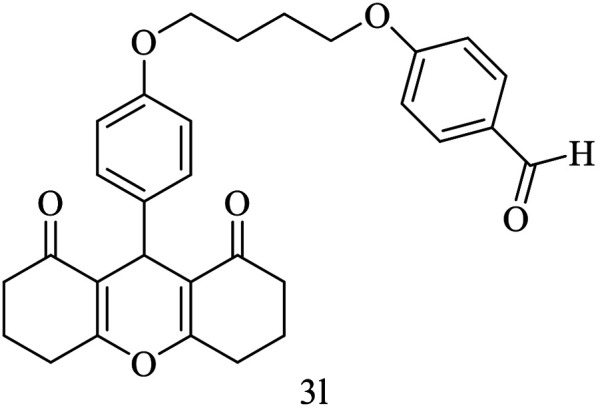
|
15 | 90 | 168–170 | New |
| 13 |
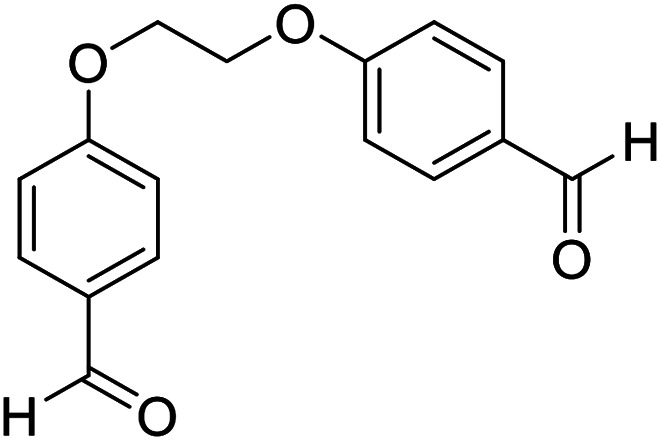
|
Me |
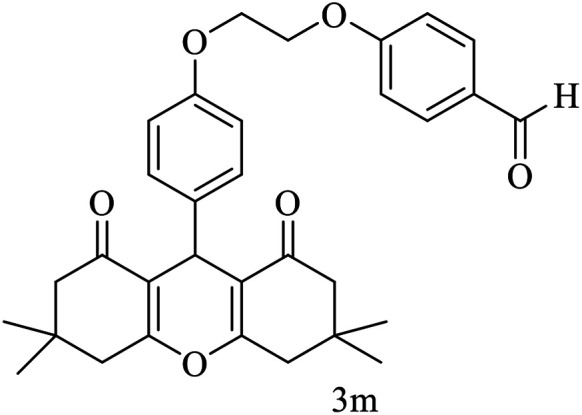
|
15 | 92 | 200–202 | New |
| 14 |
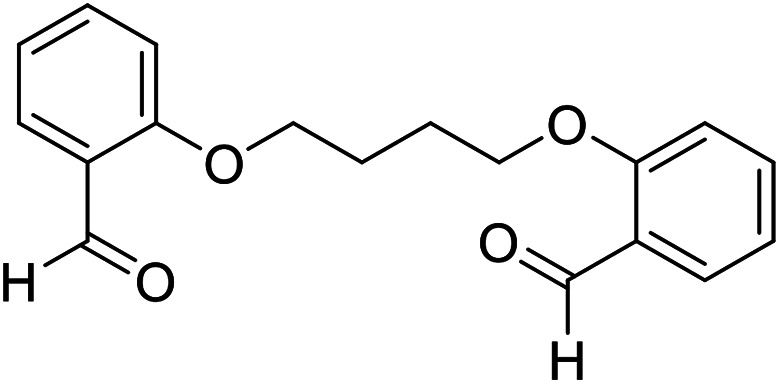
|
H |
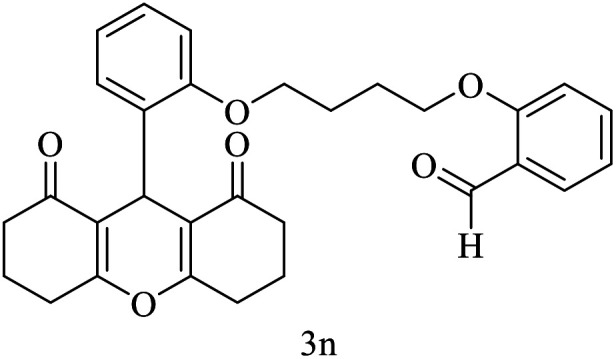
|
15 | 92 | 220–222 | New |
| 15 |
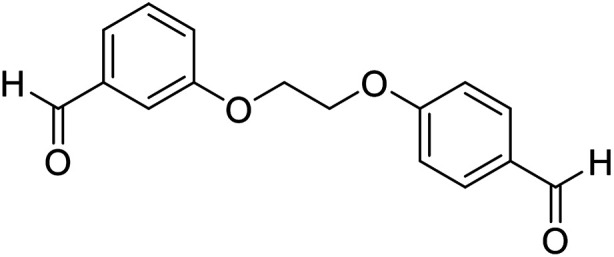
|
Me |
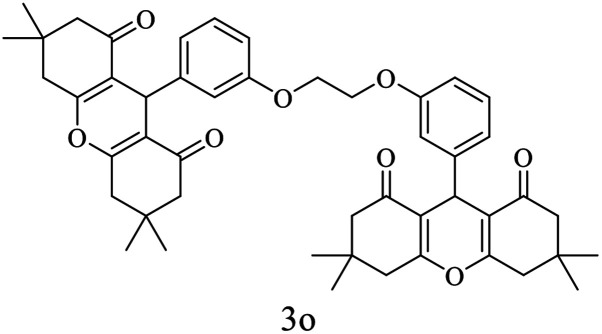
|
20 | 95 | 250–252 | New |
Reaction conditions: benzaldehyde (1.0 mmol), dimedone (2.0 mmol) and catalyst (0.5 mol%) and EtOH (3 ml), 45 °C.
Isolated yield.
