Reductive reaction of enamine 11 to racemates 13a.
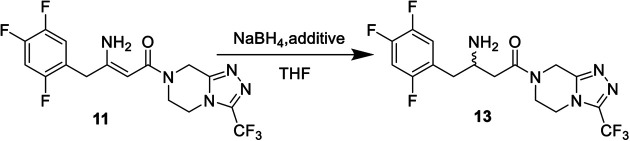
| |||
|---|---|---|---|
| Entry | Reduction | Additive | Conversion (%) |
| 1 | NaBH4 | None | 0 |
| 2 | NaBH4 | BF3 diethyl etherate | 95 |
| 3 | NaBH4 | MsOH (methanesulfonic acid) | 93 |
| 4 | NaBH4 | Acetic acid | 10 |
| 5 | NaBH4 | TFA (trifluoroacetic acid) | 2 |
All reactions were carried out as follows: THF (50 mL) was firstly cooled to −10 °C. NaBH4 (2.33 g, 61.68 mmol) and an additive such as MsOH (5.9 g, 61.68 mmol) were added dropwise at −10 to −5 °C, and then enamine 11 (5.0 g, 12.34 mmol) and isopropanol (30 mL) were added. The reaction mixture was aged at −15 °C for 4.5 h, monitored by HPLC. Isolated yield after ethyl acetate extraction.
