Introduction
Mutations in SCN5A, which encodes the voltage-gated Nav1.5 channel, cause a variety of genetic arrhythmia syndromes, including long QT syndrome type 3,1 Brugada syndrome,2 sinus node dysfunction,3 atrial fibrillation,4 progressive conduction system disease,5 dilated cardiomyopathy,6 and overlap syndromes.7 Recently, a syndrome characterized by multifocal ectopic Purkinje potential contractions (MEPPC) has been described.8 We present here 2 unrelated patients with nearly identical clinical presentations of initially incessant atrial and subsequently ventricular tachycardia (VT), carrying the same novel SCN5A mutation. We believe that this represents a new SCN5A-related entity.
Case report
Case 1
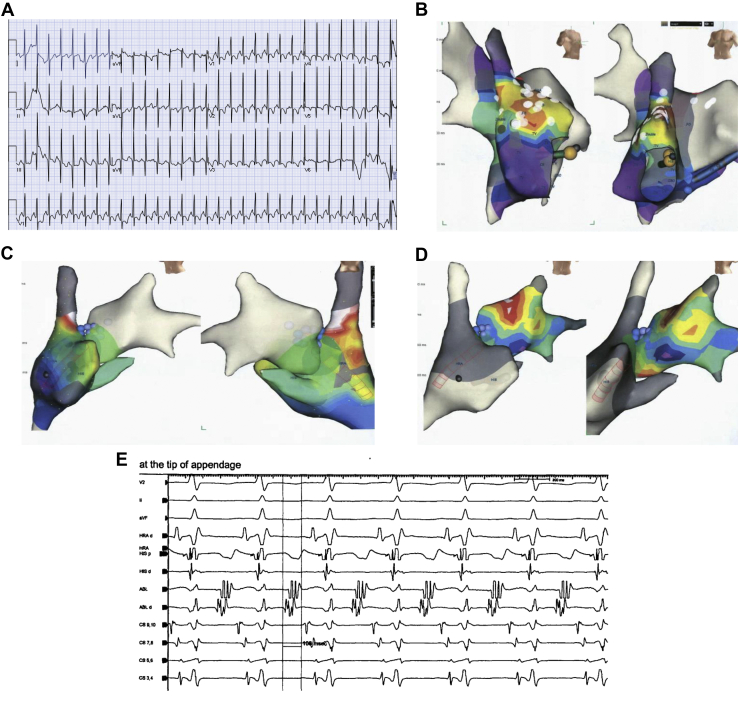
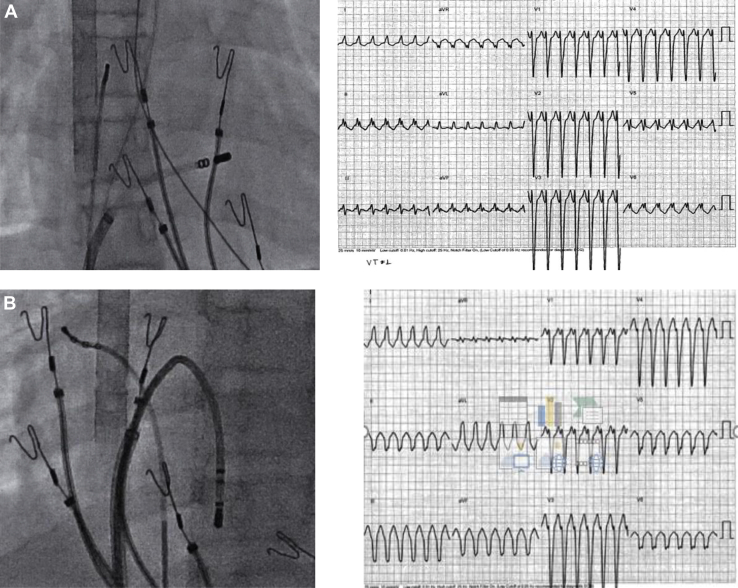
A now 16-year-old female patient of Asian-Pacific origin presented at 20 months of age with persistent ectopic atrial tachycardia (EAT) at 180 beats per minute (bpm), with narrow QRS (Figure 1A). Various antiarrhythmic medications including propranolol, digoxin, sotalol, flecainide, and combinations of the above at therapeutic doses were ineffective. Amiodarone was started at 6 years of age and achieved rate control at 100–110 bpm, but with persistent EAT. Between 6 and 10 years of age, she underwent 4 unsuccessful attempts at radiofrequency ablation by 3 operators. During these procedures, she had multiple ectopic foci in both right and left atria, including the right atrial appendage, limbus of fossa ovalis, superior vena cava/right atrial junction, left side of atrial septum, roof of left atrium, and left upper pulmonary vein. Figure 1Β–1D demonstrates various locations of EAT foci by electroanatomic mapping and Figure 1E shows intracardiac electrograms from 1 of the EAT locations. Each ablation attempt resulted in acceleration of the tachycardia followed by a shift to a different focus. She remained in incessant EAT, without any periods of sinus rhythm. Βecause of a decrease in her diffusion capacity (68% of predicted), amiodarone was discontinued and sotalol and verapamil were started without success. Her left ventricular (LV) function, which was previously normal, started to decline (LV ejection fraction [LVEF] of 38%). In the meantime, her electrocardiogram changed to right bundle branch block with AV dissociation. This was initially misdiagnosed as junctional ectopic tachycardia with right bundle branch block, but later diagnosed as VT. A trial of ivabradine in addition to sotalol and verapamil was followed by hemodynamic collapse and requirement of extracorporeal membrane oxygenation (ECMO). Two attempts were made to ablate her VT, 1 during ECMO support and 1 after decannulation, but again, during all ablation attempts, tachycardia accelerated and moved to a different focus without termination. Various morphologies of tachycardia and locations of ablation attempts are shown in Figure 2A–2C. In at least 1 of the locations in anterior RV, Purkinje potentials were recorded prior to the QRS (Figure 2D).
Figure 1.
A: Twelve-lead electrocardiogram of ectopic atrial tachycardia at presentation with narrow QRS (60 ms). B–D: Electroanatomic maps during atrial tachycardia showing earliest atrial activation in the right atrial appendage (B), superior vena cava–right atrium junction (C), and roof of left atrium (D). E: Intracardiac electrograms during one of the ablation attempts in the right atrial appendage.
Figure 2.
Radiographic images, 12-lead electrocardiograms, and intracardiac electrograms during ventricular tachycardia showing various morphologies and ablation location attempts. A: Anterior right ventricle (RV) wall. B: RV apex. C: Left ventricular septum. D: Purkinje-like potentials preceding the QRS onset at a site in the anterior RV.
She was successfully weaned from ECMO. She had transient termination of tachycardia with brief asystole and a dual-chamber pacemaker was implanted. After discussion with an expert panel (Sudden Arrhythmic Death Syndrome advisory board), quinidine was started while continuing propranolol at 3 mg/kg/day. She initially responded well to quinidine at a dose of 100 mg every 6 hours (10 mg/kg/day), with resultant AV sequential paced rhythm, and remained stable for 7 months after discharge from the hospital. She was noted to have recurrence of EAT and VT and her LVEF started to decline. Her quinidine dose was gradually increased to 300 mg every 6 hours with a quinidine level of 2.7 mcg/mL (therapeutic range 2–5 mcg/mL). She remained clinically stable until she presented with an episode of apparent cardiac arrest, requiring cardiopulmonary resuscitation. Interrogation of her pacemaker did not reveal any high-rate episodes. She was placed in the transplant list and underwent successful orthotopic heart transplantation 3 weeks later.
The explanted heart had normal size, with mild cellular hypertrophy and mild interstitial fibrosis, without significant inflammation. The previous sites of ablation were noted in both ventricles and in the right atrial appendage. There was moderate hypertrophy of the right atrium.
Genetic testing identified a heterozygous likely pathogenic mutation in SCN5A [c.2816T>C (p.Leu939Pro)]. Both parents were negative for this variant.
Case 2
A now 20-year-old male patient of Greek origin presented at 3.5 months of age with incessant EAT, with heart rate of 218 bpm, QRS duration 80 ms, and normal cardiac anatomy and ventricular function. Several medications were tried, including digoxin, propranolol, propafenone, sotalol, amiodarone, and verapamil, alone and in various combinations, at therapeutic doses without complete success. The combination of amiodarone and verapamil achieved rate control with a mean heart rate of 120 bpm. He underwent 3 ablations at 3, 7, and 8 years of age at 2 institutions, during which multiple ectopic atrial foci were found (roof and posterior wall of left atrium and right upper pulmonary vein). Each time a radiofrequency application was made, there was acceleration of the tachycardia with a change in focus, without actual termination of tachycardia. He was maintained on amiodarone and propranolol with LV function at the lower normal range. At 15 years of age, he presented with incessant VT, which was resistant to intravenous lidocaine and procainamide, and he underwent 2 unsuccessful ablations at 15 and 16 years of age. During both of these procedures, 3 different morphologies with early foci were found at the base of both LV papillary muscles and the inferior septum, with early Purkinje-like potentials preceding QRS onset by as much as 38 ms in some of these sites. Despite multiple applications with standard and irrigated ablation catheters, tachycardia could not be terminated, but there was shifting to different foci.
He was discharged on amiodarone, flecainide, and propranolol, with decreased ventricular rate, but incessant tachycardia. He developed tachycardia-induced cardiomyopathy with an LV ejection fraction of 19%. Amiodarone was discontinued because of hyperthyroidism. It was decided to attempt treatment with quinidine. After 2 doses of hydroquinidine 300 mg, his tachycardia stopped and he remained in sinus rhythm with first-degree AV block. Several days later, he was found to be in slow VT again despite treatment with hydroquinidine 300 mg 4 times per day and propranolol 40 mg 3 times per day. Amiodarone was added at 100 mg per day and an implantable defibrillator was placed. He subsequently remained in normal sinus rhythm with first-degree AV block and his echocardiogram 6 months later demonstrated a normal LVEF of 56%.
He underwent genetic testing, which demonstrated a heterozygous likely pathogenic mutation in SCN5A [c.2816T>C (p.Leu939Pro)]. Parents were not genetically tested.
Functional studies
The L939P mutation was introduced into an SCN5A expression plasmid,9 and wild-type (WT) and L939P plasmids were transiently transfected into Chinese hamster ovary cells and studied by manual whole-cell patch clamping, as previously described.10 Compared to WT, L939P showed a ∼75% decrease in peak current density at -30 mV (119.5 ± 16.7 pA/pF for WT vs 30.3 ± 4.2 pA/pF for L939P, P < .01, Figure 3). In addition, L939P had a marked increase in late current at -30 mV (0.2% ± 0.04% of peak current for WT vs 6.1% ± 1.6% of peak current for L939P, P < .01, Supplemental Figure 1A–1C); even uncorrected for peak current, the late current size for L939P was significantly increased (127.5 ± 33.9 pA vs WT 7.5 ± 1.5 pA, P < .01). Treatment with a late current blocker GS-967 completely eliminated the late current (Supplemental Figure 1D). Thus, L939P has pronounced loss- and gain-of-function in vitro functional features and may be considered an “overlap” mutation.11
Figure 3.
A: Representative sodium currents recorded in Chinese hamster ovary (CHO) cells transfected with human cardiac wild-type SCN5A. B: Representative sodium currents recorded in CHO cells transfected with L939P. C: Summary of the current-voltage relationships of the activations of the 2 groups of sodium current. The voltage-clamp protocol is shown as an insert.
Our further study of the effect of quinidine on the mutation L939P found that quinidine at 10 μM (within its therapeutic concentrations) reduced both peak and late currents generated by L939P (Supplemental Figure 1E). However, quinidine suppressed late current more than peak current (Supplemental Figure 1F): late current reduced by 76% ± 6% vs peak current reduced by 56% ± 13%.
Discussion
The 2 patients we described, although totally unrelated, have remarkable similarities:
-
(1)
Early onset of incessant atrial tachycardias in the first 2 years of life.
-
(2)
Clinical picture dominated initially by ectopic atrial tachycardia for several years, with later onset of incessant VT, leading to tachycardia-induced cardiomyopathy.
-
(3)
Resistance to multiple antiarrhythmic drugs, responding finally to quinidine, but requiring addition of amiodarone for complete control of tachycardia in 1 of the patients.
-
(4)
Resistance to multiple ablation attempts both for the atrial tachycardia and the VT
-
(5)
Identical mutation in the SCN5A gene.
SCN5A L939P is a novel variant, absent from the genome aggregation database (gnomAD)12 and, to our knowledge, from the cardiac genetics literature.13 Our patients did not have typical features of the previously described arrhythmia syndromes related to SCN5A.1, 2, 3, 4, 5, 6, 7, 8 Specifically, they did not have QT prolongation at baseline. They also did not have signs of Brugada syndrome, even while on sodium channel blocking drugs. Despite their incessant VT, they did not have immediately life-threatening arrhythmias (torsade des pointes or polymorphic VT, which characterize long QT syndrome and Brugada syndrome, respectively). Their arrhythmias were incessant (with no presence of sinus rhythm at any time) and not in the form of frequent ectopic activity, as is seen in the MEPPC syndrome.8,14 However, they share some features with the MEPPC syndrome, which include the multifocal nature of the ventricular arrhythmias, which appeared to arise from the Purkinje fibers. Unlike some of the patients with the MEPPC syndrome,8 our patients did not respond to flecainide, but they had an immediate response to quinidine.8,14 However, as the amiodarone effect waned, arrhythmias reappeared in both of our patients, and addition of a small dose of amiodarone in 1 of our patients resulted in excellent control. Quinidine has also been reported to be effective in other arrhythmias related to Purkinje system irritation, such as after catheter ablation, myocardial infarction, or coronary revascularization procedures.15
In regard to the ventricular dysfunction, this was most likely related to incessant VT, at least in the second patient, as it resolved completely upon adequate arrhythmia control. However, the first patient developed an episode of sudden severe ventricular dysfunction requiring ECMO support, and she also had an episode of out-of-hospital cardiac arrest, not explained by any tachyarrhythmia based on interrogation of her pacemaker. It is possible that the second episode was also due to sudden severe ventricular dysfunction, as the one previously documented. This second episode led us to proceed with heart transplantation, as it was thought that a defibrillator would not adequately protect her. The second patient received a defibrillator because of the possibility of a life-threatening arrhythmia, as he also had a very low ejection fraction at that time.
We would like to emphasize the complete lack of response to catheter ablation of either the atrial or the ventricular arrhythmias. This is in accordance with the reports of MEPPC in the literature.8,14 Even though at any time a single morphology of tachycardia was present, whenever an early atrial or ventricular focus was tried there was acceleration of the tachycardia and switching to a different focus. At several of the ventricular sites there were Purkinje potentials at the early sites, but there was never even transient tachycardia termination.
As far as the underlying genetic abnormality is concerned, the 2 patients carry an identical mutation in SCN5A (Leu929Pro) with a mixed effect: loss of function in the peak sodium current and gain-of-function effect in the late sodium current.11 The effect in the late sodium current is more significant and may thus explain the efficacy of quinidine, which had a much more significant effect on the late current.
Conclusion
We believe that we have described a new genetic arrhythmia syndrome related to SCN5A that is characterized by incessant atrial and later onset of VT, resistant to all antiarrhythmic medications and ablation and responsive to quinidine. Awareness of this entity will help other clinicians to make timely therapeutic decisions and avoid potential worsening of ventricular function and dangerous complications of multiple medications and catheter interventions.
Key Teaching Points.
-
•
In patients with incessant atrial and/or ventricular arrhythmias resistant to multiple medications, genetic evaluation should be performed to inform etiology and guide management.
-
•
Mutations in the SCN5A gene may have mixed effects on the peak and late sodium currents, with implications for choice of antiarrhythmic therapy.
-
•
In arrhythmias related to the Purkinje system, failing other antiarrhythmic medications and/or catheter ablation, treatment with quinidine should be strongly considered.
Footnotes
Conflicts of Interest: John Papagiannis, Tao Yang, Andrew M. Glazer, Svjetlana Tisma-Dupanovic, Dimosthenis Avramidis, Prince J. Kannankeril, Sami Viskin, and Dan M. Roden report no conflicts of interest. Edward P. Walsh: Up-to-Date (author honorarium), Biosense Webster (divisional research funds, no personal remuneration).
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hrcr.2021.08.013.
Appendix. Supplementary data
References
- 1.Wilde A.A.M., Moss A.J., Kaufman E.S., et al. Clinical aspects of type 3 long-QT syndrome: an international multicenter study. Circulation. 2016;134:872–882. doi: 10.1161/CIRCULATIONAHA.116.021823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brugada P., Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome. A multicenter report. J Am Coll Cardiol. 1992;20:1391–1396. doi: 10.1016/0735-1097(92)90253-j. [DOI] [PubMed] [Google Scholar]
- 3.Benson D.W., Wang D.W., Dyment M., et al. Congenital sick sinus syndrome caused by recessive mutations in the cardiac sodium channel gene (SCN5A) J Clin Invest. 2003;112:1019–1028. doi: 10.1172/JCI18062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Darbar D., Kannankeril P.J., Donahue B.S., et al. Cardiac sodium channel (SCN5A) variants associated with atrial fibrillation. Circulation. 2008;117:1927–1935. doi: 10.1161/CIRCULATIONAHA.107.757955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Probst V., Kyndt F., Potet F., et al. Haploinsufficiency in combination with aging causes SCN5A-linked hereditary Lenègre disease. J Am Coll Cardiol. 2003;41:643–652. doi: 10.1016/s0735-1097(02)02864-4. [DOI] [PubMed] [Google Scholar]
- 6.McNair W.P., Ku L., Taylor M.R.G., et al. Familial Cardiomyopathy Registry Research Group. SCN5A mutation associated with dilated cardiomyopathy, conduction disorder, and arrhythmia. Circulation. 2004;110:2163–2167. doi: 10.1161/01.CIR.0000144458.58660.BB. [DOI] [PubMed] [Google Scholar]
- 7.Clancy C.E., Rudy Y. Na (+) channel mutation that causes both Brugada and long-QT syndrome phenotypes: a simulation study of mechanism. Circulation. 2002;105:1208–1213. doi: 10.1161/hc1002.105183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laurent G., Saal S., Amarouch M.Y., et al. Multifocal ectopic Purkinje-related premature contractions: a new SCN5A-related cardiac channelopathy. J Am Coll Cardiol. 2012;60:144–156. doi: 10.1016/j.jacc.2012.02.052. [DOI] [PubMed] [Google Scholar]
- 9.Glazer A.M., Wada Y., Li B., et al. High-throughput reclassification of SCN5A variants. Am J Hum Genet. 2020;107:111–123. doi: 10.1016/j.ajhg.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang T., Chun Y.W., Stroud D.M., et al. Screening for acute IKr block is insufficient to detect torsades de pointes liability: role of late sodium current. Circulation. 2014;130:224–234. doi: 10.1161/CIRCULATIONAHA.113.007765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Remme C.A., Wilde A.A., Bezzina C.R. Cardiac sodium channel overlap syndromes: different faces of SCN5A mutations. Trends Cardiovasc Med. 2008;18:78–87. doi: 10.1016/j.tcm.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Karczewski K.J., Francioli L.C., Tiao G., et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. 2020;581:434–443. doi: 10.1038/s41586-020-2308-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kroncke B.M., Glazer A.M., Smith D.K., Blume J.D., Roden D.M. SCN5A (NaV1.5) variant functional perturbation and clinical presentation: variants of a certain significance. Circ Genom Precis Med. 2018;11 doi: 10.1161/CIRCGEN.118.002095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doisne N., Waldmann V., Redheuil A., et al. A novel gain-of-function mutation in SCN5A responsible for multifocal ectopic Purkinje-related premature contractions. Hum Mutat. 2020;41:850–859. doi: 10.1002/humu.23981. [DOI] [PubMed] [Google Scholar]
- 15.Viskin S., Chorin E., Viskin D. Quinidine-responsive polymorphic ventricular tachycardia in patients with coronary heart disease. Circulation. 2019;139:2304–2314. doi: 10.1161/CIRCULATIONAHA.118.038036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.