Abstract
Objective
To clarify the effect of postmastectomy radiotherapy (PMRT) on pT1-2N1 breast cancer patients with different molecular subtypes.
Methods
We retrospectively analyzed the data of 5442 patients with pT1-2N1 breast cancer treated using modified radical mastectomy in 11 hospitals in China. Univariate, multivariate, and propensity score matching (PSM) analyses were used to evaluate the effect of PMRT on locoregional recurrence (LRR).
Results
With a median follow-up duration of 63.8 months, the 5-year LRR rates were 4.0% and 7.7% among patients treated with and without PMRT, respectively (p < 0.001). PMRT was independently associated with reduced LRR after adjustments for confounders (p < 0.001). After grouping the patients according to the molecular subtype of cancer and conducting PSM, we found that the 5-year LRR rates among patients treated with and without PMRT (in that order) were as follows: luminal HER2-negative cancer, 1.9% and 6.5% (p < 0.001); luminal HER2-positive cancer, 3.8% and 13.7% (p = 0.041); HER2-overexpressing cancer, 10.2% and 15.5% (p = 0.236); and triple-negative cancer, 4.6% and 15.9% (p = 0.002). Among patients with HER2-overexpressing and triple-negative cancers, the LRR hazard rate displayed a dominant early peak, and was extremely low after 5 years. However, patients with luminal cancer continued to have a long-lasting high annual LRR hazard rate during follow-up.
Conclusion
PMRT significantly reduced the LRR risk in patients with pT1-2N1 luminal and triple-negative breast cancers, but had no effect on the LRR risk in patients with HER2-overexpressing cancer. Patients with different molecular subtypes displayed different annual LRR patterns, and the late recurrence of the luminal subtype suggests the necessity of long-term follow-up to evaluate the efficacy of PMRT.
Keywords: Breast neoplasm, Modified radical mastectomy, Radiotherapy, Molecular subtype, 1–3 positive lymph nodes
Highlights
-
•
PMRT reduces LRR of pT1-2N1 luminal and triple-negative breast cancers.
-
•
PMRT has no effect on the LRR of HER2-overexpressing breast cancer.
-
•
Different molecular subtypes display different annual LRR patterns.
-
•
Late LRR beyond 5 years is observed in luminal breast cancers.
1. Introduction
Breast cancer is the most common cancer among women worldwide [1]. Postoperative radiotherapy (RT) can significantly reduce the locoregional recurrence (LRR) rate and breast cancer mortality in high-risk patients who have undergone mastectomy [2]. The 2020 National Comprehensive Cancer Network guidelines (3rd edition) [3] recommend that patients with ≥4 positive axillary lymph nodes after total mastectomy should undergo postoperative RT, and patients with 1–3 positive axillary lymph nodes (i.e., N1) should strongly consider postoperative RT. The 2019 St. Gallen International Consensus Guidelines [4] recommend postoperative RT for stage N1 patients with triple-negative breast cancer, but opinions differ on the need for postoperative RT in patients with human epidermal growth factor receptor 2 (HER2)-positive and/or estrogen receptor (ER)-positive cancers. The purpose of this study was to analyze the effect of RT on LRR in patients with different molecular subtypes of pT1-2N1 stage breast cancer after modified radical mastectomy as well as to analyze the annual LRR patterns in this patient population to obtain evidence for making decisions on adjuvant RT.
2. Materials and methods
2.1. Patient population
This study was approved by the institutional review board of Cancer Hospital, Chinese Academy of Medical Sciences (approval number: 15–057/984). As this study is a retrospective analysis of chart data, the need for informed consent was waived. The data of women with pT1-2N1 breast cancer treated at 11 hospitals in China between September 1997 and December 2014 were retrospectively analyzed. The inclusion criteria were as follows: (1) newly diagnosed breast cancer with a pathological tumor size of ≤5 cm after modified radical mastectomy and metastasis to 1–3 axillary lymph nodes (pT1-2N1); (2) no metastasis to the supra- or infraclavicular or internal mammary lymph nodes at initial diagnosis; (3) no distant metastasis at initial diagnosis; and (4) no neoadjuvant therapy. The following exclusion criteria were applied: (1) unknown adjuvant RT status; (2) simultaneous bilateral breast cancer; (3) unknown date of surgery; and (4) unknown date of last follow-up.
2.2. Follow-up and prognosis evaluation criteria
Follow-up was conducted by means of outpatient visits, inpatient visits, or telephone calls. The timing of all events was measured from the date of modified radical mastectomy to the date of event occurrence or the last follow-up. Prognosis was evaluated using indicators such as LRR, distant metastasis (DM), disease-free survival (DFS), and overall survival (OS). LRR was defined as recurrence in the ipsilateral chest wall and/or the supra- or infraclavicular fossa, axilla, or internal mammary region. DM was defined as recurrence at sites other than those indicating LRR. DFS was defined as the time from the date of surgery to the date of LRR, DM, death, or the last follow-up. OS was defined as the time from the date of surgery to the date of death or the last follow-up.
2.3. Statistical analysis
LRR and survival rates were calculated using the Kaplan-Meier method and compared using the log-rank test. The association of LRR with potential prognostic factors was tested using univariate Cox regression analysis and further evaluated using multivariable proportional hazards regression for the entire cohort and for patients with different molecular subtypes of breast cancer. To minimize differences in the distribution of covariates between the RT and no RT groups for patients with different molecular subtypes, we used propensity score matching (PSM). PSM was performed after taking into consideration all possible relevant factors in the analysis. The matching approach was 1:1 nearest neighbor matching within a caliper distance of 10%. Statistical analyses were performed using the SPSS v26.0 software (IBM SPSS Statistics for Windows, version 26.0.; IBM Corp., Armonk, NY). A p-value of ≤0.05 was considered statistically significant. The “muhaz package” in R v4.0.1 (R Project for Statistical Computing, Austria, Vienna; www.r-project.org/) was used to calculate the annual LRR risk.
3. Results
3.1. Baseline characteristics
In all, we reviewed the data of 5504 patients, of whom, 62 patients were excluded because of unknown adjuvant RT date (n = 2), simultaneous bilateral breast cancer (n = 1), unknown date of surgery (n = 2), and unknown date of last follow-up (n = 57). Thus, a total of 5442 patients were included in the study. The median age was 49 years (range, 20–84 years). The median number of lymph nodes removed was 16 (range, 1–59), and the median number of positive lymph nodes was 1 (range, 1–3). Other baseline characteristics are shown in Table 1.
Table 1.
Baseline characteristics of 5442 pT1-2N1 breast cancer patients who underwent modified radical mastectomy.
| Variable | Number of patients (%) |
|---|---|
| Treatment era | |
| 1997–2007 | 1911 (35.1) |
| 2008–2014 | 3531 (64.9) |
| Age (years) | |
| ≤40 | 957 (17.6) |
| >40 | 4485 (82.4) |
| Tumor location | |
| Inner quadrants | 1142 (21.0) |
| Other quadrants | 4090 (75.1) |
| Unknown | 210 (3.9) |
| Pathological type | |
| Invasive ductal carcinoma | 5090 (93.5) |
| Others | 351 (6.4) |
| Unknown | 1 (0.1) |
| pT stage | |
| pT1 | 2387 (43.9) |
| pT2 | 3055 (56.1) |
| Number of positive nodes | |
| 1 | 2725 (50.1) |
| 2 | 1673 (30.7) |
| 3 | 1044 (19.2) |
| Number of nodes removed | |
| <10 | 585 (10.7) |
| ≥10 | 4857 (89.3) |
| Tumor grade | |
| I | 131 (2.4) |
| II | 2939 (54.0) |
| III | 1146 (21.1) |
| Unknown | 1226 (22.5) |
| Lymphovascular invasion | |
| Yes | 607 (11.2) |
| No | 4525 (83.1) |
| Unknown | 310 (5.7) |
| Hormone receptors | |
| Positive | 4150 (76.3) |
| Negative | 1183 (21.7) |
| Unknown | 109 (2.0) |
| HER2 status | |
| Positive | 1045 (19.2) |
| Negative | 3575 (65.7) |
| Unknown | 822 (15.1) |
| Ki67 index | |
| <14% | 1698 (31.2) |
| ≥14% | 1456 (26.8) |
| Unknown | 2288 (42.0) |
| Molecular subtype | |
| Luminal HER2-negative | 2919 (53.6) |
| Luminal HER2-positive | 645 (11.9) |
| HER2-overexpressing | 400 (7.4) |
| Triple-negative | 654 (12.0) |
| Unknown | 824 (15.1) |
| Endocrine therapya (n = 4150) | |
| Yes | 3452 (83.2) |
| No | 466 (11.2) |
| Unknown | 232 (5.6) |
| Anti-HER2-targeted therapyb (n = 1045) | |
| Yes | 190 (18.2) |
| No | 813 (77.8) |
| Unknown | 42 (4.0) |
| Adjuvant chemotherapy | |
| Yes | 5118 (94.0) |
| No | 278 (5.1) |
| Unknown | 46 (0.8) |
| Adjuvant radiotherapy | |
| Yes | 1780 (32.7) |
| No | 3662 (67.3) |
Only patients with hormone receptor-positive cancer were analyzed.
Only patients with HER2-positive cancer were analyzed.
Among the 5442 patients, 4150 patients had hormone receptor-positive tumors, of whom, 3452 (83.2%) patients underwent endocrine therapy. The principal endocrine therapy regimens were tamoxifen/toremifene (40.9%), aromatase inhibitor (25.9%), ovarian function suppression (OFS) + tamoxifen/toremifene (2.0%), and OFS + aromatase inhibitor (0.7%). In addition, two different types of these endocrine therapy regimens were successively applied in 5.6% of patients. In all, 1045 patients had HER2-positive tumors; however, because trastuzumab was approved by the China Food and Drug Administration in September 2007, only 190 (18.2%) of these patients underwent anti-HER2 targeted therapy. The anti-HER2 targeted drugs used were trastuzumab (175 patients, 92.1%) and lapatinib (4 patients, 2.1%); for 11 (5.8%) patients, the drug used for targeted therapy was unknown. Adjuvant chemotherapy was administered to 5118 (94.0%) patients, for a median of 6 cycles (range, 1–21 cycles). Adjuvant RT was delivered to 1780 (32.7%) patients. Conventional fractionated RT was used for adjuvant RT in 1603 (90.1%) patients, and the median dose was 50 Gy in 25 fractions. Hypofractionated RT was used for 52 (2.9%) patients, with a median dose of 40 Gy in 15 fractions. The dose fractionation was unknown in 125 (7.0%) patients. The chemotherapy regimens and RT targets in the entire cohort and in patients with different molecular subtypes of breast cancer are summarized in Table 2. Most patients received two-dimensional RT with a linear accelerator at a dose rate of 600 MU/min. In brief, the chest wall was irradiated using a 6–9 MeV electron beam, depending on the chest wall thickness and the dose prescribed to the Dmax. A 5-mm tissue equivalent bolus, up to a median of 40% of the total prescribed dose, was also applied. The supraclavicular nodal region was irradiated using one anterior-posterior field and the dose was prescribed to the isopoint at 3 cm beneath the skin.
Table 2.
Chemotherapy regimens and radiation targets in the entire cohort and in patients with different molecular subtypes of breast cancer.
| Entire cohort | Luminal HER2-negative | Luminal HER2-positive | HER2-overexpressing | Triple-negative | Unknown subtype | |
|---|---|---|---|---|---|---|
| Chemotherapy regimen (N = 5442) | ||||||
| Anthracycline-based | 1126 (20.7) | 618 (21.2) | 87 (13.5) | 70 (17.5) | 143 (21.9) | 208 (25.2) |
| Taxane-based | 301 (5.5) | 144 (4.9) | 43 (6.7) | 10 (2.5) | 60 (9.2) | 44 (5.3) |
| Both | 3245 (59.6) | 1772 (60.7) | 440 (68.2) | 284 (71.0) | 375 (57.3) | 374 (45.4) |
| Other | 204 (3.7) | 77 (2.6) | 8 (1.2) | 7 (1.8) | 24 (3.7) | 88 (10.7) |
| Unknown | 566 (10.4) | 308 (10.6) | 67 (10.4) | 29 (7.2) | 52 (8.0) | 110 (13.3) |
| Chemotherapy cycles | ||||||
| median (range) | 6 (1–21) | 6 (1–20) | 6 (1–9) | 6 (1–21) | 6 (1–13) | 6 (1–10) |
| RT target (N = 1780) | ||||||
| Chest wall | 1642 (92.2) | 799 (93.3) | 188 (91.3) | 136 (92.5) | 234 (95.5) | 283 (87.4) |
| Supra/infraclavicular fossa | 1612 (90.6) | 773 (90.3) | 179 (86.9) | 135 (91.8) | 229 (93.5) | 296 (90.8) |
| Axilla | 146 (8.2) | 46 (5.4) | 12 (5.8) | 9 (6.1) | 22 (9.0) | 57 (17.5) |
| Internal mammary chain | 162 (9.1) | 36 (4.2) | 17 (8.3) | 8 (5.4) | 15 (6.1) | 86 (26.4) |
| Unknown | 84 (4.7) | 43 (2.4) | 13 (0.7) | 4 (0.2) | 5 (0.3) | 19 (1.1) |
RT, radiotherapy.
3.2. Effect of radiotherapy on recurrence, metastasis, and survival rates in the entire cohort
The median follow-up period for the entire cohort was 63.8 months (range, 0.2–228.8 months). A total of 496 patients died, including 388 (78.2%) patients who died of breast cancer, 4 (0.8%) patients who died of complications of breast cancer treatment, 60 (12.1%) patients who died of other causes unrelated to breast cancer, and 44 (8.9%) patients who died of unknown causes. There were a total of 1013 cases of tumor recurrence or metastasis, including 395 cases (39.0%) of LRR and 731 cases (72.2%) of DM.
The 5-year OS, DFS, LRR, and DM rates of the entire cohort were 93.1%, 83.9%, 6.6%, and 11.7%, respectively. Compared to patients who did not undergo RT, patients who underwent RT had a higher 5-year OS (93.8% vs. 92.6%, hazard ratio [HR] = 0.68, 95% confidence interval [CI]: 0.55–0.84, p < 0.001), a higher 5-year DFS (84.4% vs. 83.5%, HR = 0.84, 95% CI: 0.73–0.96, p = 0.012), and a lower 5-year LRR rate (4.0% vs. 7.7%, HR = 0.50, 95% CI: 0.38–0.64, p < 0.001). However, there was no significant difference in the 5-year DM rate between these two groups (12.3% vs. 11.5%, HR = 0.97, 95% CI: 0.82–1.14, p = 0.690; Fig. 1). After adjustments for confounders, multivariate analyses showed that RT independently reduced LRR (p < 0.001; Table 3).
Fig. 1.
Recurrence and survival curves of 5442 breast cancer patients who did or did not undergo postmastectomy radiotherapy (PMRT). (a) Overall survival (OS); (b) disease-free survival (DFS); (c) locoregional recurrence (LRR); and (d) distant metastasis (DM).
Table 3.
Multivariate analyses of variables associated with LRR in the entire cohort and in patients with different molecular subtypes of breast cancer.
| Variable |
P value |
|||||
|---|---|---|---|---|---|---|
| Entire cohort | Luminal HER2-negative | Luminal HER2-positive | HER2- overexpressing | Triple- negative | Unknown subtype | |
| Treatment era 1997–2007 vs. 2008–2014 | 0.915 | 0.571 | 0.774 | 0.931 | 0.592 | 0.133 |
| ≤40 vs. >40 years old | 0.003 | 0.096 | 0.447 | 0.911 | 0.839 | 0.096 |
| Inner quadrants vs. others | <0.001 | 0.003 | 0.681 | 0.530 | 0.154 | 0.002 |
| pT1 vs. pT2 | <0.001 | <0.001 | 0.384 | 0.799 | 0.007 | 0.064 |
| <10 vs. ≥10 nodes removed | 0.084 | 0.344 | 0.641 | 0.864 | 0.145 | 0.094 |
| 1 vs. 2–3 positive nodes | 0.001 | 0.048 | 0.082 | 0.602 | 0.005 | 0.054 |
| G1-2 vs. G3 | 0.002 | 0.147 | 0.467 | 0.094 | 0.814 | 0.454 |
| LVI + vs. LVI- | 0.147 | 0.249 | 0.387 | 0.044 | 0.672 | 0.981 |
| RT vs. no RT | <0.001 | <0.001 | 0.010 | 0.821 | 0.001 | 0.002 |
| Chemotherapy vs. no chemotherapy | 0.260 | 0.474 | 0.416 | 0.550 | 0.967 | 0.961 |
| Endocrine therapy vs. no endocrine therapy | 0.001 | 0.239 | 0.957 | 0.081 | – | 0.453 |
| Anti-HER2- targeted therapy vs. no targeted therapy | 0.992 | 0.973 | 0.935 | 0.515 | – | 0.993 |
LRR, locoregional recurrence; LVI, lymphovascular invasion; RT, radiotherapy.
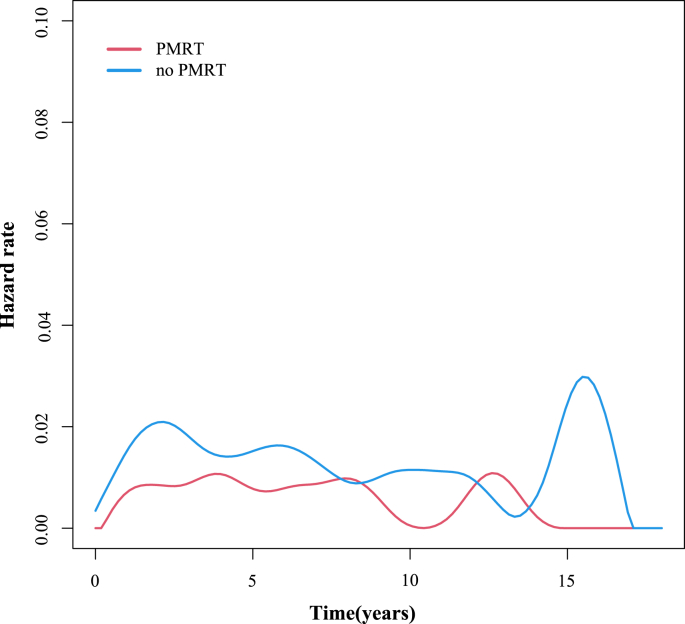
The annual LRR risk curve of the entire cohort showed that the annual LRR risk of patients who underwent RT remained at a low level, about 1%/year. In contrast, the LRR risk curve of patients who did not undergo RT exhibited two peaks, at about 2 years and 15 years after the surgery (Fig. 2).
Fig. 2.
Annual locoregional recurrence risk curve of 5442 breast cancer patients who did or did not undergo postmastectomy radiotherapy (PMRT).
3.3. Effect of radiotherapy on LRR in patients with different molecular subtypes of breast cancer
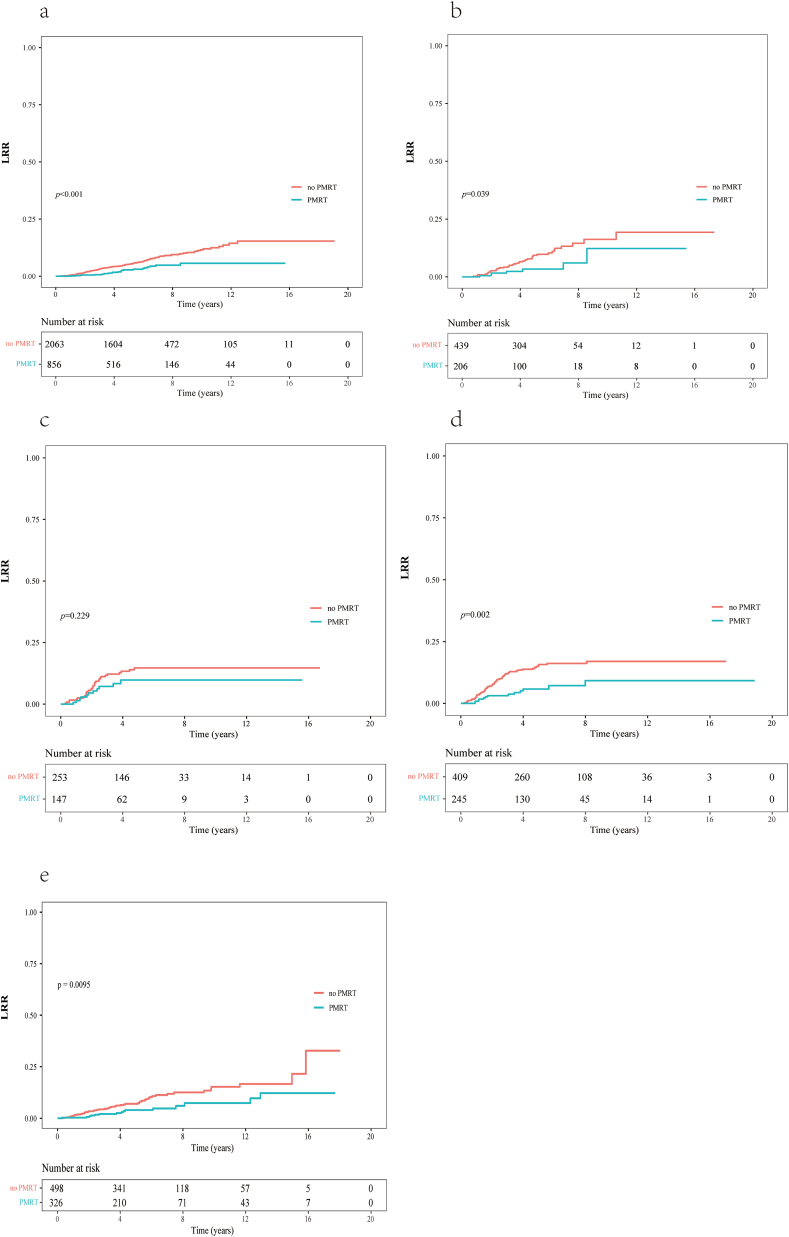
The molecular subtype was known in 4618 patients and unknown in 824 patients (Table 1). A total of 29.3% (856/2919) of patients in luminal-HER2 negative, 31.9% (206/645) of patients in luminal-HER2 positive patients, 36.8% (147/400) of patients in HER2-overexpressing patients, 37.5% (245/654) of patients in triple-negative patients, and 39.6% (326/824) of patients in unknown subtype underwent RT. In each of the above 5 subgroups, the 5-year LRR rates among patients who did or did not undergo RT (in that order) were as follows: luminal HER2-negative cancer, 2.8% and 5.3% (HR = 0.45, 95% CI: 0.29–0.70, p < 0.001); luminal HER2-positive cancer, 3.4% and 9.3% (HR = 0.44, 95% CI: 0.20–0.98, p = 0.039); HER2-overexpressing cancer, 9.8% and 14.7% (HR = 0.66, 95% CI: 0.33–1.31, p = 0.229); triple-negative cancer, 5.8% and 15.7% (HR = 0.40, 95% CI: 0.22–0.73, p = 0.002); and unknown subtype, 4.0% and 7.4% (HR = 0.47, 95% CI: 0.29–0.78, p = 0.009; Fig. 3). In the HER2-overexpressing cancer subgroup, 64 patients underwent anti-HER2-targeted therapy. Among these 64 patients, the 5-year LRR rates of those who did or did not undergo RT were 4.0% and 7.7%, respectively (HR = 0.55, 95% CI: 0.06–5.30, p = 0.630). Among the 320 patients in the HER2-overexpressing group who did not undergo anti-HER2-targeted therapy, the 5-year LRR rates of those who did or did not undergo RT were 13.2% and 15.2%, respectively (HR = 0.86, 95% CI: 0.42–1.73, p = 0.687).
Fig. 3.
Locoregional recurrence (LRR) curves of patients with different molecular subtypes of breast cancer who did or did not undergo postmastectomy radiotherapy (PMRT). (a) Luminal HER2-negative cancer; (b) luminal HER2-positive cancer; (c) HER2-overexpressing cancer; (d) triple-negative cancer; and (e) unknown molecular subtype.
After adjustments for confounders, multivariate analyses showed that RT significantly reduced the LRR rate in patients with luminal HER2-negative cancer (p < 0.001), luminal HER2-positive cancer (p = 0.010), triple-negative cancer (p = 0.001), and unknown molecular subtype (p = 0.002). However, RT had no effect on LRR in patients with HER2-overexpressing cancer (p = 0.821; Table 3).
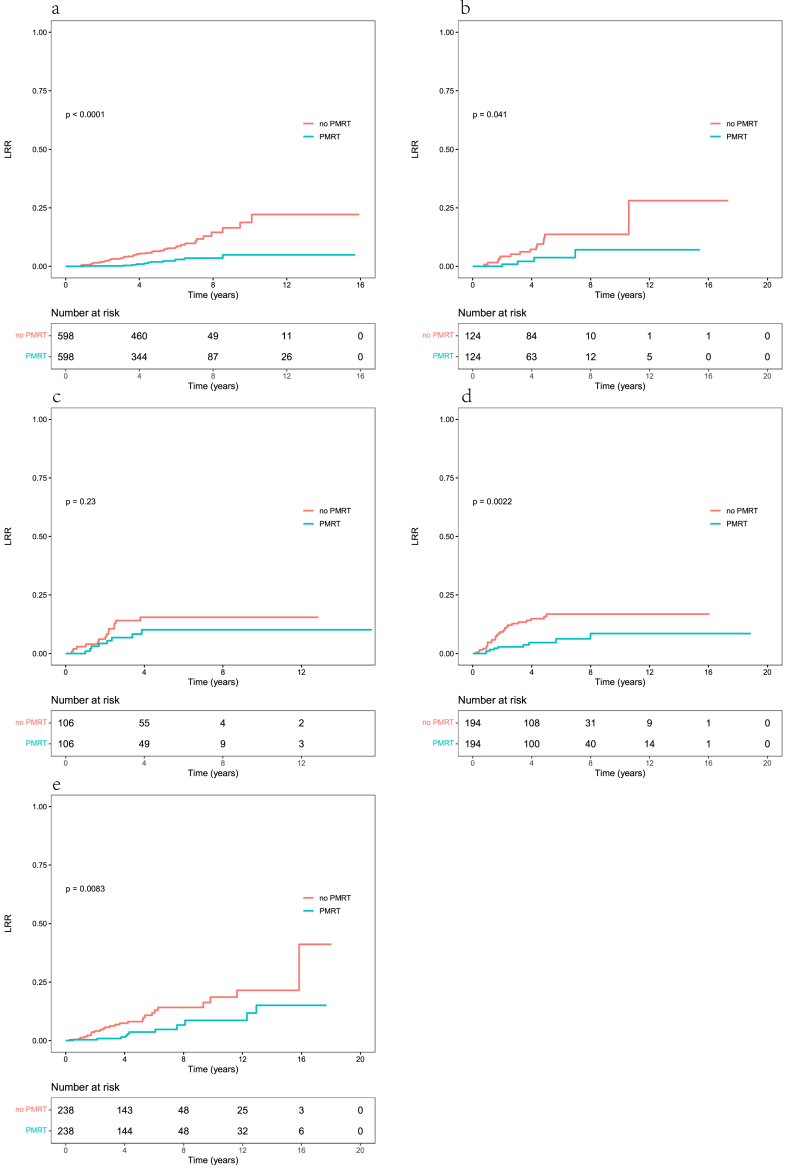
After PSM, the confounders were well balanced between patients who received RT and those who did not in the five subgroups based on molecular subtypes (Supplementary Tables S1–S5). The 5-year LRR rates in patients with and without RT (in that order) were as follows: 1.9% and 6.5% in the luminal HER2-negative subgroup (HR = 0.24, 95% CI: 0.12–0.46, p < 0.001), 3.8% and 13.7% in the luminal HER2-positive subgroup (HR = 0.33, 95% CI: 0.11–0.99, p = 0.041), 10.2% and 15.5% in the HER2-overexpressing subgroup (HR = 0.59, 95% CI: 0.25–1.42, p = 0.236), 4.6% and 15.9% in the triple-negative subgroup (HR = 0.33, 95% CI: 0.16–0.69, p = 0.002), and 3.7% and 8.1% in the unknown subtype subgroup (HR = 0.40, 95% CI: 0.20–0.81, p = 0.008; Fig. 4).
Fig. 4.
Locoregional recurrence (LRR) curves of patients with different molecular subtypes of breast cancer who did or did not undergo postmastectomy radiotherapy (PMRT) after propensity score matching. (a) Luminal HER2-negative cancer; (b) luminal HER2-positive cancer; (c) HER2-overexpressing cancer; (d) triple-negative cancer; and (e) unknown molecular subtype.
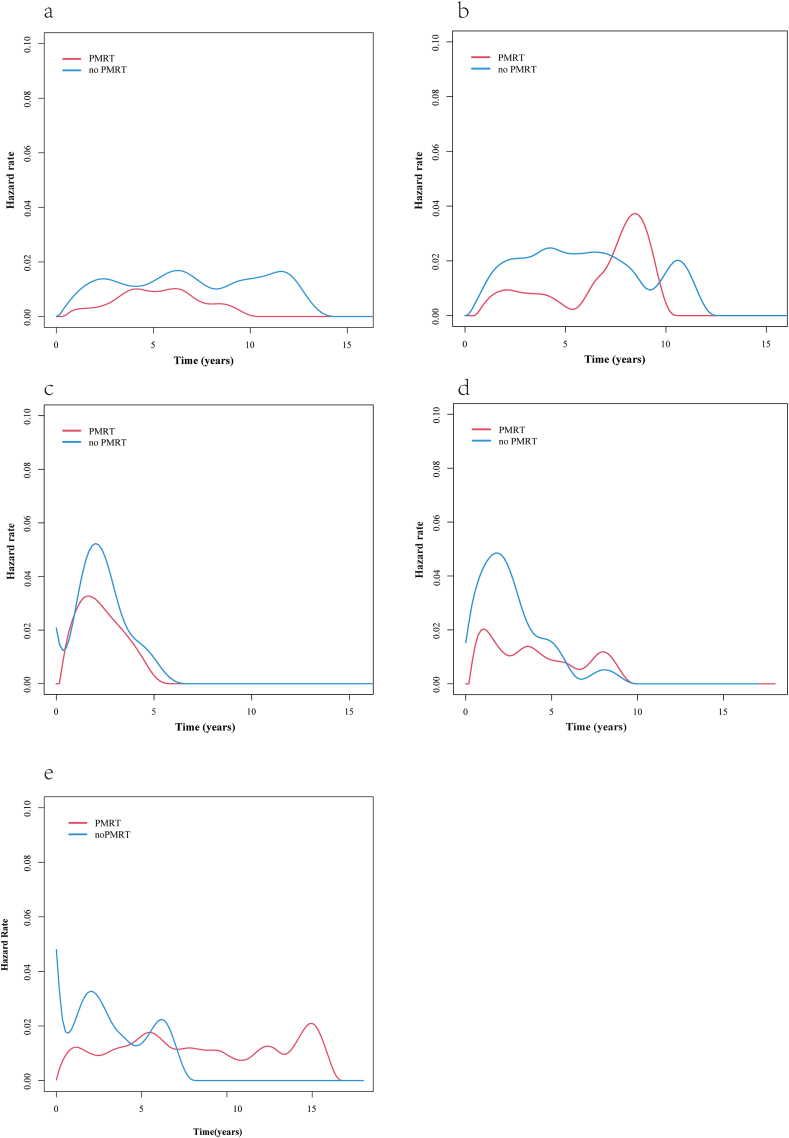
We next analyzed the annual LRR risk curves for patients with different molecular subtypes (Fig. 5). Among patients with luminal HER2-negative cancer, the annual LRR risk curve of those who underwent RT showed a single peak at about 5 years after the surgery, and that the annual LRR risk was always lower for those who underwent RT than for those who did not undergo RT (Fig. 5a). In the luminal HER2-positive subgroup, both patients who underwent RT as well as those who did not undergo RT had 2 peaks in their annual LRR risk curves: a low and flat first peak at about 2 and 4 years after the surgery, respectively, and a second peak at 8 and 11 years after the surgery, respectively (Fig. 5b). In the HER2-overexpressing subgroup, both patients who underwent RT and those who did not undergo RT had a single peak in their annual LRR risk curve, at about 2 years after the surgery. The maximum annual LRR risk was slightly lower in patients who underwent RT than in patients who did not undergo RT (Fig. 5c). In the triple-negative cancer subgroup, the annual LRR risk curve of patients who did not undergo RT showed a single peak at about 2 years after the surgery, and these patients had an LRR risk of about 5%/year. The annual risk curve of triple-negative cancer patients who did undergo RT showed 3 peaks, at about 1, 4, and 8 years after the surgery. The maximum risk was at 1 year after the surgery, and the overall LRR risk was about 2%/year (Fig. 5d).
Fig. 5.
Annual locoregional recurrence (LRR) risk curves of patients with different molecular subtypes of breast cancer who did or did not undergo postmastectomy radiotherapy (PMRT). (a) Luminal HER2-negative cancer; (b) luminal HER2-positive cancer; (c) HER2-overexpressing cancer; (d) triple-negative cancer; and (e) unknown molecular subtype.
4. Discussion
The analyses of patients with stage pT1-2N1 breast cancer in the present study revealed that postmastectomy RT (PMRT) significantly reduced the risk of LRR in the entire cohort and in patients with the luminal or triple-negative cancer subtypes, but did not significantly reduce this risk in patients with the HER2-overexpressing cancer subtype. RT changed the annual LRR pattern in the entire cohort, and the annual LRR risk differed between patients with different molecular subtypes of breast cancer.
Studies have shown that RT has different effects on breast cancers with different molecular subtypes. It is generally agreed that RT can significantly reduce the risk of LRR in patients with luminal breast cancer and can even improve their OS. In contrast, the effects of RT on patients with HER2-overexpressing or triple-negative breast cancer are inconsistent. The DBCG 82 b & c study reported the same findings as the present study, namely, that PMRT significantly reduces the risk of LRR in patients with luminal and triple-negative breast cancers and has no effect on LRR in patients with HER2-overexpressing breast cancer [5]. The results of two early randomized clinical trials [6] showed that PMRT significantly reduced LRR in patients with the luminal A subtype, but the effect of PMRT on patients with other molecular subtypes could not be analyzed in these trials due to the small sample sizes. An analysis of T1-2N1 breast cancer using the SEER database [7] showed that PMRT significantly improved the OS rate of patients with luminal A breast cancer, but had no effect on the OS of patients with luminal B, HER2-overexpressing, and triple-negative breast cancers. An analysis of 774 breast cancer patients with 4 or more positive lymph nodes by Wu et al. [8] showed that PMRT significantly improved the local recurrence-free survival (LRFS) of patients with luminal A and luminal B breast cancers, but had no effect on the LRFS of patients with HER2-overexpressing and triple-negative breast cancers. Tseng et al. [9] analyzed patients with stage I–III breast cancer, and found that PMRT was most able to reduce LRR in patients with luminal A cancer, followed by those with luminal B cancer and those with HER2-overexpressing breast cancer who underwent anti-HER2-targeted therapy; PMRT did not significantly reduce LRR in patients with triple-negative breast cancer. Basic research has confirmed that HER2-positive breast cancer cells are resistant to radiation, and that anti-HER2-targeted therapy can reverse the radiation resistance of HER2-positive cells. Pietras et al. [10] reported that MCF-7 cells transfected with HER2 were more resistant to radiation than parental MCF-7 cells, and anti-HER2-targeted therapy could reverse the radiation resistance of HER2-overexpressing cell lines by regulating the repair of radiation-induced DNA damage. In the present study, RT did not significantly reduce LRR in patients with HER2-overexpressing cancer, regardless of whether they underwent anti-HER2-targeted therapy. Moreover, RT did not significantly reduce LRR in patients who underwent anti-HER2-targeted therapy. These results may be related to the small sample size.
The annual LRR risk curve of the patients in this study indicated the presence of a late recurrence peak at 15 years after the surgery among those who did not undergo RT, suggesting that this group of patients requires long-term follow-up. A study by Woodward et al. [11] had a median follow-up period of 14 years, and found that the 10-year LRR rates of patients with stage pT1-2N1 breast cancer who did or did not undergo RT after mastectomy were 3% and 13%, respectively (p = 0.003). A study by McBride et al. [12] included patients with stage pT1-2N1 breast cancer after mastectomy treated in the early (1978–1997) and later (2000–2007) treatment eras. PMRT significantly reduced the 15-year LRR rate in the early era cohort (6.1% vs. 14.5%, p = 0.035); in the later era cohort, there was no significant difference in the 5-year LRR rates between patients who did and did not undergo PMRT (2.8% vs. 4.2%, p = 0.48). The long follow-up period for the early era cohort showed that PMRT significantly reduced LRR. However, with advancements in diagnoses and treatments, these results may not be suitable for current clinical practice.
Further analysis in the present study showed that patients with different molecular subtypes have different annual LRR patterns. The LRRs of patients with HER2-overexpressing and triple-negative cancers were primarily distributed within 5 years after the surgery, suggesting that 5-year follow-up results can be used to guide RT decisions in these patients. However, patients with luminal cancers and patients with unknown molecular subtypes (of whom 94.6% were hormone receptor-positive) had a high risk of late LRR, suggesting that long-term follow-up results beyond 5 years are needed for evaluating the efficacy of RT in these patients. Other studies on the recurrence patterns in patients with different molecular subtypes have yielded similar findings. Demicheli et al. found that patients with ER-negative and ER-positive cancers have different annual recurrence patterns. Patients with ER-negative cancer have an obvious early recurrence peak, whereas patients with ER-positive cancer show a slow increase in early recurrence risk and a continuous and gradually decreasing low-level recurrence risk at later time points [13]. Ribelles et al. [14] reported that patients with the luminal subtype had a long tail of recurrence after 5 years, and patients with triple-negative breast cancer exhibited early unimodal recurrence, whereas the pattern for patients with HER2-overexpressing cancer was complex. Since most patients did not undergo anti-HER2-targeted therapy, the recurrence pattern of patients with Ki-67 ≥ 14% was similar to that of patients with triple-negative cancer, while those with Ki-67 < 14% had two recurrence peaks, at 2 and 6 years after treatment. Sun et al. [15] found that anti-HER2-targeted therapy changed the annual LRR risk pattern of patients with HER2-positive breast cancer. Without anti-HER2-targeted therapy, patients had bimodal recurrences at 2.5 and 9 years after treatment; with anti-HER2-targeted therapy, they had only a single recurrence peak at 2.5 years after treatment.
This study has some limitations. First, it is a retrospective study; there was selection bias toward patients undergoing RT, and there is a possibility of underestimating LRR events. Second, the time span of the patient enrollment was as long as 20 years, and advances in diagnosis and treatment during this time period may cause the findings to be unable to reflect current clinical practice. Finally, the number of cases with long-term follow-up for over 10 years was low, which might affect the reliability of the late recurrence results.
In summary, the present study shows that PMRT significantly reduced the risk of LRR in stage pT1-2N1 luminal and triple-negative breast cancer patients, but the effect of PMRT in patients with HER2-overexpressing cancer was not statistically significant. Patients with breast cancers of different molecular subtypes have different annual LRR patterns, and late recurrence in patients with the luminal subtype suggests the need for long-term follow-up to evaluate the efficacy of RT.
Author contributions
Xin-yuan Guo, Hong-Mei Wang, Min Liu: formal analysis, investigation, data collection, methodology, and writing of the first draft. Guang-yi Sun: data collection, statistical analysis, and editing of the revised manuscript. Shu-lian Wang, Mei Shi, and Jing Cheng: formal analysis and data collection, validation, statistical guidance, project administration, patient care, and writing and editing of the first draft of the manuscript. Yu-jing Zhang: patient care, data collection, and review and editing of the manuscript. Na Zhang: patient care, data collection, and review and editing of the manuscript. Yu Tang: patient care, data collection, and review and editing of the manuscript. Xu-ran Zhao: patient care, data collection, and review and editing of the manuscript. Ge Wen: patient care, data collection, and review and editing of the manuscript. Qi-shuai Guo: patient care, data collection, and review and editing of the manuscript. Hong-fen Wu: patient care, data collection, and review and editing of the manuscript. Xiao-hu Wang: patient care, data collection, and review and editing of the manuscript. Chang-yin Ma: patient care, data collection, and review and editing of the manuscript. Ye-xiong Li: patient care, data collection, and review and editing of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the CAMS Innovation Fund for Medical Sciences (2020-I2M-C&T-B-075), the National Natural Science Foundation of China (81972860), and the Medical Scientific Research Foundation of Guangdong Province, China (B2020065).
Data availability
All datasets presented in this study are included in the article.
Declaration of competing interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Not applicable.
Footnotes
Xin-Yuan Guo, Guang-Yi Sun, Hong-Mei Wang, and Min Liu contribute equally as co-first authors.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2021.12.013.
Contributor Information
Xin-Yuan Guo, Email: 1157931131@qq.com.
Jing Cheng, Email: chenjin1118@hotmail.com.
Mei Shi, Email: mshi82@fmmu.edu.cn.
Shu-Lian Wang, Email: wsl20040118@yahoo.com.
Abbreviations
- RT
Radiotherapy
- LRR
Locoregional recurrence
- HER2
Human epidermal growth factor receptor 2
- ER
Estrogen receptor
- DM
Distant metastasis
- DFS
Disease-free survival
- OS
Overall survival
- PMRT
Postmastectomy radiotherapy
- LRFS
Local recurrence-free survival
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.McGale P., Taylor C., Correa C., Cutter D., Duane F., Ewertz M., Gray R., Mannu G., Peto R., Whelan T., Wang Y., Wang Z., Darby S. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet (Lond Engl) 2014;383:2127–2135. doi: 10.1016/S0140-6736(14)60488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gradishar W.J., Anderson B.O., Abraham J., Aft R., Agnese D., Allison K.H., Blair S.L., Burstein H.J., Dang C., Elias A.D., Giordano S.H., Goetz M.P., Goldstein L.J., Isakoff S.J., Krishnamurthy J., Lyons J., Marcom P.K., Matro J., Mayer I.A., Moran M.S., Mortimer J., O'Regan R.M., Patel S.A., Pierce L.J., Rugo H.S., Sitapati A., Smith K.L., Smith M.L., Soliman H., Stringer-Reasor E.M., Telli M.L., Ward J.H., Young J.S., Burns J.L., Kumar R. Breast cancer, version 3.2020, NCCN clinical practice guidelines in Oncology. J Natl Compr Cancer Netw. 2020;18:452–478. doi: 10.6004/jnccn.2020.0016. [DOI] [PubMed] [Google Scholar]
- 4.Burstein H.J., Curigliano G., Loibl S., Dubsky P., Gnant M., Poortmans P., Colleoni M., Denkert C., Piccart-Gebhart M., Regan M., Senn H.J., Winer E.P., Thurlimann B. Estimating the benefits of therapy for early-stage breast cancer: the St. Gallen International Consensus Guidelines for the primary therapy of early breast cancer 2019. Ann Oncol. 2019;30:1541–1557. doi: 10.1093/annonc/mdz235. [DOI] [PubMed] [Google Scholar]
- 5.Kyndi M., Sørensen F.B., Knudsen H., Overgaard M., Nielsen H.M., Overgaard J. Estrogen receptor, progesterone receptor, HER-2, and response to postmastectomy radiotherapy in high-risk breast cancer: the Danish Breast Cancer Cooperative Group. J Clin Oncol. 2008;26:1419–1426. doi: 10.1200/JCO.2007.14.5565. [DOI] [PubMed] [Google Scholar]
- 6.Laurberg T., Tramm T., Nielsen T., Alsner J., Nord S., Myhre S., Sørlie T., Leung S., Fan C., Perou C., Gelmon K., Overgaard J., Voduc D., Prat A., Cheang M.C.U. Intrinsic subtypes and benefit from postmastectomy radiotherapy in node-positive premenopausal breast cancer patients who received adjuvant chemotherapy - results from two independent randomized trials. Acta Oncol (Stockh) 2018;57:38–43. doi: 10.1080/0284186X.2017.1401735. [DOI] [PubMed] [Google Scholar]
- 7.Wei J., Jiang Y., Shao Z. The survival benefit of postmastectomy radiotherapy for breast cancer patients with T1-2N1 disease according to molecular subtype. Breast. 2020;51:40–49. doi: 10.1016/j.breast.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu S.G., He Z.Y., Li Q., Li F.Y., Lin Q., Lin H.X., Guan X.X. Predictive value of breast cancer molecular subtypes in Chinese patients with four or more positive nodes after postmastectomy radiotherapy. Breast. 2012;21:657–661. doi: 10.1016/j.breast.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 9.Tseng Y.D., Uno H., Hughes M.E., Niland J.C., Wong Y.N., Theriault R., Blitzblau R.C., Moy B., Breslin T., Edge S.B., Hassett M.J., Punglia R.S. Biological subtype predicts risk of locoregional recurrence after mastectomy and impact of postmastectomy radiation in a large national database. Int J Radiat Oncol Biol Phys. 2015;93:622–630. doi: 10.1016/j.ijrobp.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 10.Pietras R.J., Poen J.C., Gallardo D., Wongvipat P.N., Lee H.J., Slamon D.J. Monoclonal antibody to HER-2/neureceptor modulates repair of radiation-induced DNA damage and enhances radiosensitivity of human breast cancer cells overexpressing this oncogene. Cancer Res. 1999;59:1347–1355. [PubMed] [Google Scholar]
- 11.Woodward W.A., Strom E.A., Tucker S.L., Katz A., McNeese M.D., Perkins G.H., Buzdar A.U., Hortobagyi G.N., Hunt K.K., Sahin A., Meric F., Sneige N., Buchholz T.A. Locoregional recurrence after doxorubicin-based chemotherapy and postmastectomy: implications for breast cancer patients with early-stage disease and predictors for recurrence after postmastectomy radiation. Int J Radiat Oncol Biol Phys. 2003;57:336–344. doi: 10.1016/s0360-3016(03)00593-5. [DOI] [PubMed] [Google Scholar]
- 12.McBride A., Allen P., Woodward W., Kim M., Kuerer H.M., Drinka E.K., Sahin A., Strom E.A., Buzdar A., Valero V., Hortobagyi G.N., Hunt K.K., Buchholz T.A. Locoregional recurrence risk for patients with T1,2 breast cancer with 1-3 positive lymph nodes treated with mastectomy and systemic treatment. Int J Radiat Oncol Biol Phys. 2014;89:392–398. doi: 10.1016/j.ijrobp.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 13.Demicheli R., Biganzoli E., Ardoino I., Boracchi P., Coradini D., Greco M., Moliterni A., Zambetti M., Valagussa P., Gukas I.D., Bonadonna G. Recurrence and mortality dynamics for breast cancer patients undergoing mastectomy according to estrogen receptor status: different mortality but similar recurrence. Cancer Sci. 2010;101:826–830. doi: 10.1111/j.1349-7006.2009.01472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ribelles N., Perez-Villa L., Jerez J.M., Pajares B., Vicioso L., Jimenez B., de Luque V., Franco L., Gallego E., Marquez A., Alvarez M., Sanchez-Muñoz A., Perez-Rivas L., Alba E. Pattern of recurrence of early breast cancer is different according to intrinsic subtype and proliferation index. Breast Cancer Res. 2013;15 doi: 10.1186/bcr3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun G.Y., Jing H., Wang S.L., Song Y.W., Jin J., Fang H., Liu Y.P., Ren H., Tang Y., Zhao X.R., Song Y.C., Chen S.Y., Yang Z.B., Chen B., Tang Y., Li N., Lu N.N., Qi S.N., Yang Y., Li Y.X. Trastuzumab provides a comparable prognosis in patients with HER2-positive breast cancer to those with HER2-negative breast cancer: post Hoc analyses of a randomized controlled trial of post-mastectomy hypofractionated radiotherapy. Front Oncol. 2020;10 doi: 10.3389/fonc.2020.605750. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All datasets presented in this study are included in the article.