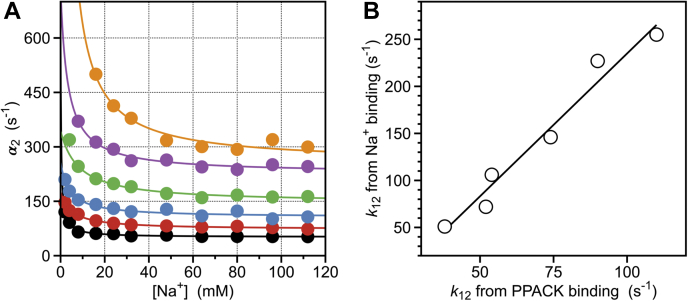
Figure 3.
Temperature dependence of Na+ binding.A, rate of relaxation for Na+ binding to thrombin over the temperature range 5 to 30 °C. Continuous lines were drawn under the rapid equilibrium approximation (26), as for the data in Figure 2B, with best-fit parameter values listed in Table 1. Experimental conditions are: 400 mM ChCl, 50 mM Tris, 0.1% PEG8000, pH 8.0 at 5 °C (black), 10 °C (red), 15 °C (cyan, see also Fig. 2A), 20 °C (green), 25 °C (purple), 30 °C (orange). B, values of the rate constant k12 measuring the transition opening access to the Na+ site (see panel A and Table 1) plotted versus the rate constant k12 measuring the transition opening access to the active site, taken from published measurements of PPACK binding under identical solution conditions (44). A strong correlation (r2 = 0.97) between the two values over the entire temperature range 5 to 30 °C supports a structural linkage between the two binding processes. A van’t Hoff plot of lnk12versus for Na+ binding is linear with an activation energy of 11 ± 1 kcal/mol, as found for PPACK binding (44). The continuous line was drawn using the expression a + bx, with best-fit parameter values: a = −68 ± 7 s−1, b = 3.0 ± 0.2.