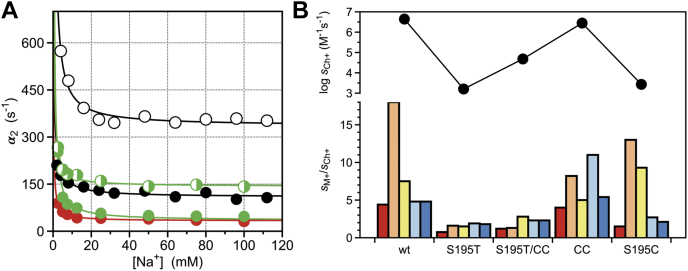
Figure 6.
S195 mutants of thrombin.A, rate of relaxation for Na+ binding to thrombin wild-type (black) and mutants S195T (white), C42A/C58A (green), C42A/C58A/S195T (shaded green), and S195C (red). Continuous lines were drawn as for the data in Figure 2B, with best-fit parameter values listed in Table 1. Experimental conditions are: 400 mM ChCl, 50 mM Tris, 0.1% PEG8000, pH 8.0, at 15 °C. B, M+ activation profile for thrombin wild-type and mutants S195T, C42A/C58A (CC), C42A/C58A/S195T (S195T/CC) and S195C. Data depict values of the specificity constant for the hydrolysis of chromogenic substrate FPR (bottom panel) relative to the value measured in the presence of the inert cation Ch+ (top panel). M+s refer to Li+ (red), Na+ (orange), K+ (yellow), Rb+ (cyan), Cs+ (blue). Experimental conditions are: 5 mM Tris, 0.1% PEG8000, pH 8.0 at 25 °C, in the presence of 400 mM M+Cl− salt as indicated.