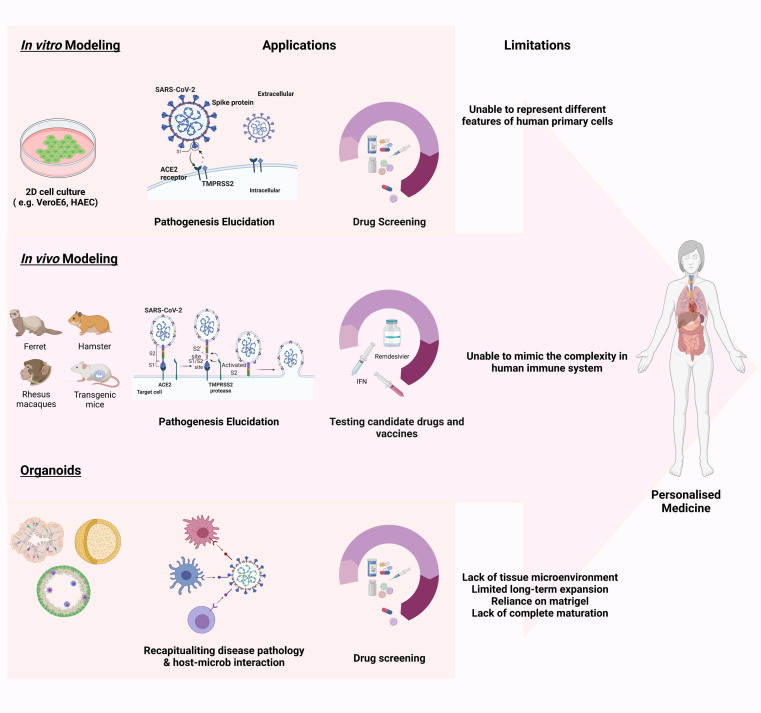
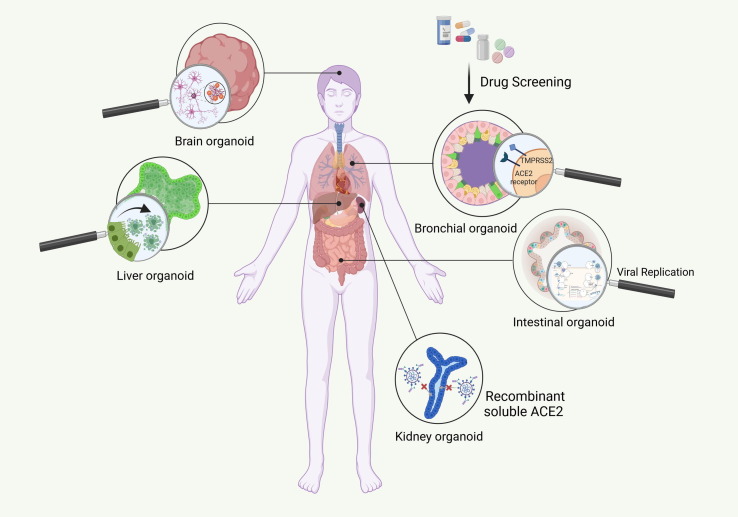
Figure 2.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) disease modeling. (a) Platforms for disease modeling. In vitro (2D adherent cultures and 3D organoids), and in vivo modeling have thus far been widely used to assess pathogenesis and test candidate drugs. In summary, Vero E6 cells and human airway epithelial cells (HAECs) are two most common cell lines used in SARS-CoV-2 studies. Genetically modified mice, hamsters, ferrets, and various nonhuman primates are among the animals used for assessing the pathogenesis and testing candidate drugs. For instance, mRNA vaccine candidates for COVID-19 have been evaluated in mice. In addition, clinical benefits of remdesivir have been shown in the Rhesus macaque model of coronavirus 2019 (COVID-19). Considering the limitations of these models, including failure to represent the multiple aspects of the infection and the inability to mimic the complex nature of human immunity, organoids are attracting attention because of their ability to show host–microbe interactions and their potential for drug screening. Organoids also have limitations such as in mimicking native tissue microenvironment, long-term culture and expansion, reliance on Matrigel, and lack of full maturity. These need to be addressed in future. (b) Organoids for mechanism elucidation in COVID-19. Given the notable manifestation ov COVID-19 in lungs, kidneys, gastrointestinal system, liver, and brain, among others, scientists have focused on creating the corresponding organoids. Thus far, human bronchial, kidney, intestinal, liver ductal, and brain organoids contain various types of cell, have been developed to mimic virus entry and recapitulate the viral life cycle, along with the possible mechanism involved in pathogenesis, including the direct cytopathic effects. Abbreviation: ACE, angiotensin-converting enzyme.