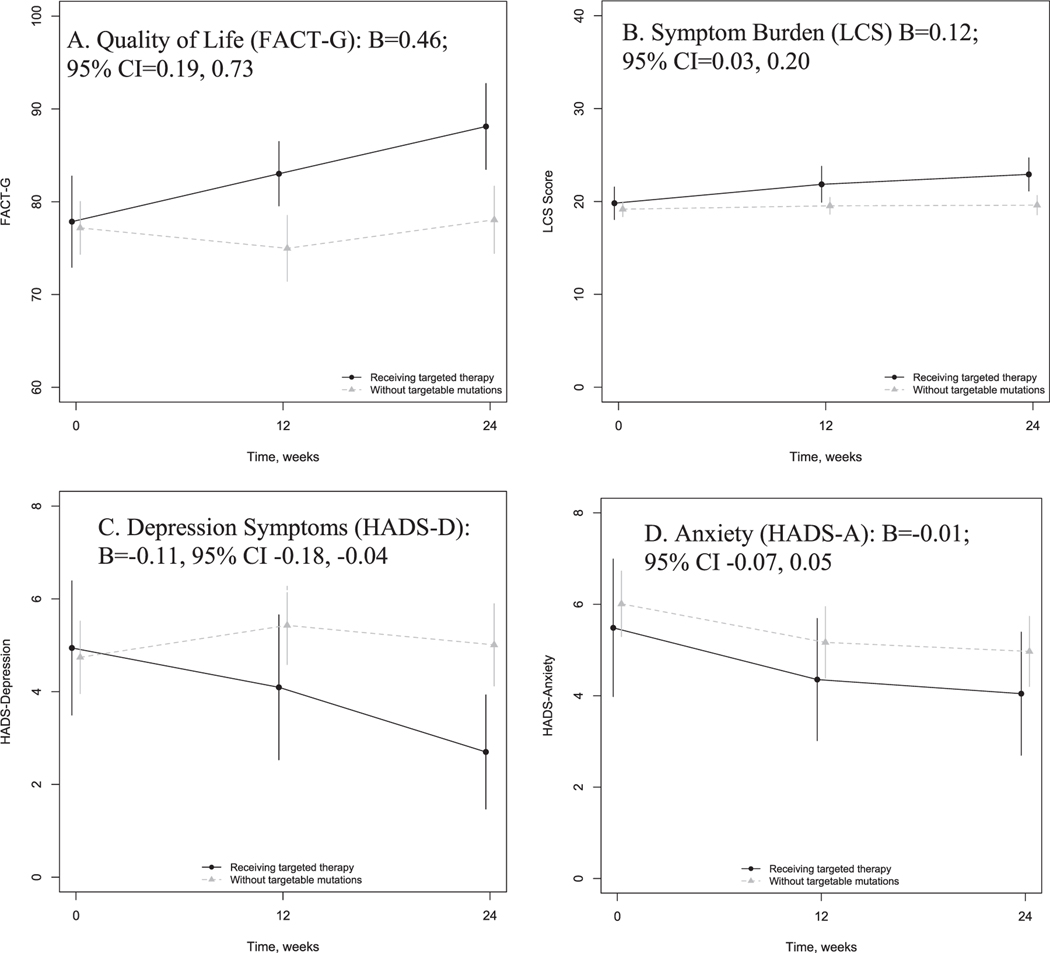
Fig. 1.
Patient-reported outcome change over time among patients receiving targeted therapy and those with lung cancer without targetable mutations. Shown are unadjusted means and 95% confidence intervals at baseline, 12, and 24 weeks for (A) Quality of life, measured with the Functional Assessment of Cancer Therapy-General (FACT-G); (B) Symptom burden, measured with the Lung Cancer Subscale (LCS) of the FACT-Lung; (C) Depression, measured by the Hospital Anxiety and Depression Scale (HADS)-Depression subscale; and (D) Anxiety, measured by the HADS-Anxiety subscale. The unstandardized coefficient of the group-time interaction from linear mixed effects models for each patient-reported outcome are also shown, with 95% confidence intervals.