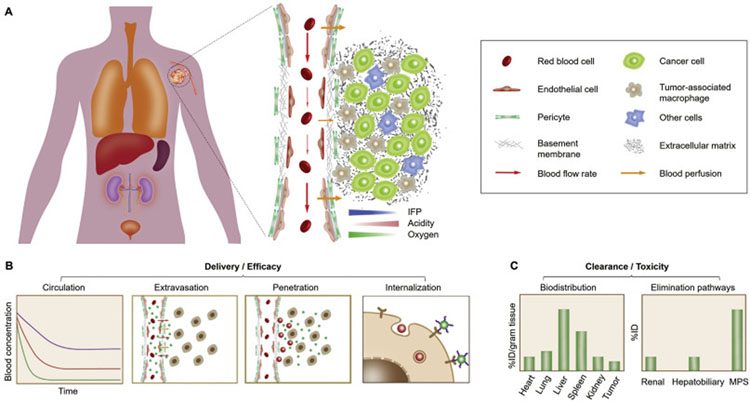
Fig. 1.
In vivo delivery and clearance of cancer nanomedicines. (A) For effective tumor targeting and delivery, the administrated nanomedicines generally take a sequential in vivo transport route, including circulation in bloodstream, extravasation across tumor vasculature, penetration within tumor interstitium to cancer cells, and cell internalization. However, a series of physiological barriers may considerably impair the therapeutic outcome of engineered nanomedicines: 1) During blood circulation, DDSs may be immediately sequestered by the mononuclear phagocyte system (MPS), resulting in shortened blood retention; 2) Subsequently, DDS extravasation across abnormal tumor vasculature is impeded by the high interstitial fluid pressure (IFP) and poor blood perfusion in tumor microenvironment (TME); 3) Next, intratumoral penetration of DDSs can be further limited due to the presence of dense extracellular matrix (ECM) and tumor-associated macrophages (TAMs); 4) Eventually, cell internalization of DDSs will be affected by their affinity not only to cancer cells but also to neighboring non-cancer cells. (B) Therapeutic efficacy of administrated nanomedicines is closely related to their delivery effectiveness to the population of cancer cells in tumor. Therefore, strategic design of nanomedicines is required to successfully overcome these physiological barriers along the in vivo transport route. (C) Meanwhile, toxicity of the administrated nanomedicines is largely affected by the different elimination pathways as well as the biodistribution and retention in normal tissues.