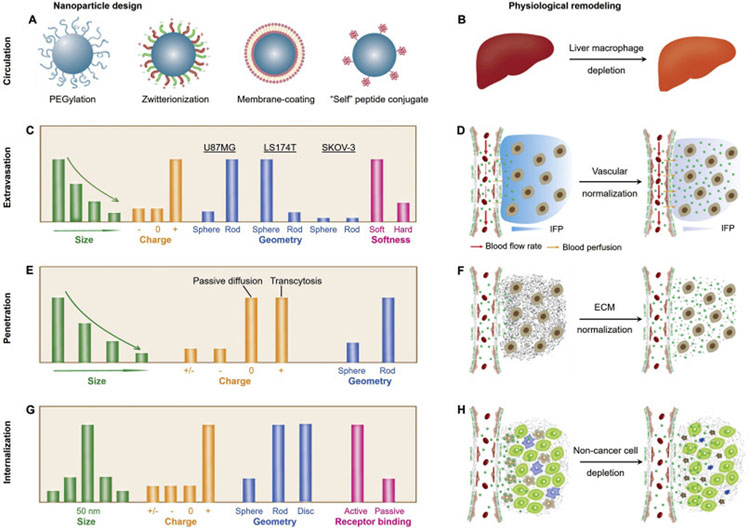
Fig. 2.
Emerging delivery strategies for various cancer nanomedicines. During the past years, many breakthroughs have been achieved in understanding the physiological barriers and developing novel delivery strategies (in nanoparticle design and physiological remodeling). (A-B) During the blood circulation, the MPS serves as one of the most important barriers for drug delivery, therefore, many strategies have been applied or proposed to escape the MPS uptake and to prolong blood circulation, including surface modification of nanomedicines (A) and depletion of liver macrophages (B). (C-D) Throughout the past decades, various physicochemical properties of NPs have been investigated to increase the vascular permeability of NPs (through EPR-based extravasation or transendothelial transport, C), whereas, normalization of tumor vasculature may also serve as a viable approach to increase the efficacy of nanomedicines (D). (E-F) The nanoparticle penetration in TME can be improved by tuning the physicochemical properties of NPs to facilitate passive diffusion or transcytosis (E) as well as by the normalization of dense and disorganized ECM (F). (G-H) After successfully overcoming the proceeding barriers, the nanomedicines that are intended for active targeting will still need to interact with cancer cells for internalization. Some strategies to potentially address this challenge includes rational nanoparticle design (in size, charge, geometry and surface conjugation, G) as well as the depletion of non-cancer cells (such as tumor-associated macrophages and fibroblasts, H).