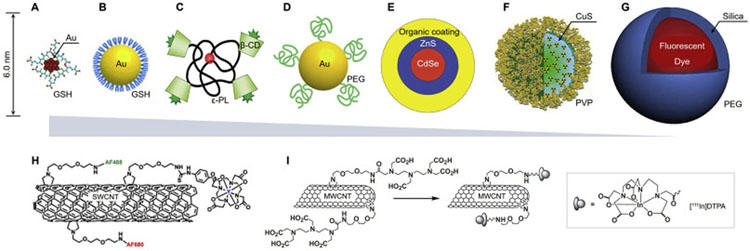
Fig. 3.
Representative engineered renal clearable NPs of various sizes and compositions. (A) Zwitterionic glutathione (GSH)-coated gold nanoclusters (Au25SG18, ~2 nm), ref. [128,174]. (B) Zwitterionic GSH-coated gold nanoparticles (GS-AuNPs, 3.3 nm), ref. [149]. (C) Organic β-cyclodextrin (β-CD)-ε-polylysine (εPL) NPs (H-dots, CDPL NPs, 4.6–4.9 nm), ref. [53]. (D) PEGylated AuNPs (PEG-AuNPs, 5.5 nm), ref. [51]. (E) Zwitterionic CdSe/ZnS quantum dots (QDs, 4.4–8.7 nm), ref. [9]. (F) Polyvinylpyrrolidone (PVP)-coated copper sulfide nanodots (CuS NDs, 5.6 nm), ref. [136]. (G) PEGylated core-shell silica-based NPs (C-dots and C′-dots, ~7 nm), ref. [127,152]. The sizes in (A-G) are reported in hydrodynamic diameter (HD). (H-I) In addition to the ultrasmall NPs (A-G), small-molecule probes and biodegradable nanomaterials, some large nanomaterials have also been reported to be renally clearable, such as single-walled carbon nanotubes (SWCNTs, average length 200–300 nm, diameter 0.8–1.2 nm, H, ref. [129]) and multi-walled carbon nanotubes (MWCNTs, length 0.5–2.0 μm, diameter 20–30 nm, I, ref. [148]). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)