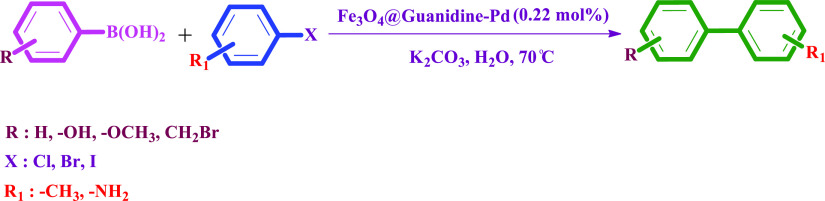
Table 2. Reaction Time, Isolated Yield, and the Melting Point of the Acquired Products in the Suzuki–Miyaura Cross-Coupling Reactiona.
| melting
point (C) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| aEntry | R | X | R1 | time (min) | yield (%) | TON | TOF (h–1) | observed | literature | ref. |
| 01 | H | I | 20 | 96 | 427 | 1295 | 69–72 | 69–71 | (51) | |
| 02 | p-OH | I | 25 | 94 | 419 | 1006 | 163–165 | 164–166 | (52) | |
| 03 | o-OCH3 | I | 45 | 85 | 378 | 505 | 87–90 | 87–89 | (52) | |
| 04 | p-CH2Br | I | 40 | 88 | 392 | 594 | 82–84 | 83–86 | (53) | |
| 05 | H | Br | 30 | 90 | 401 | 801 | 69–72 | 69–71 | (51) | |
| 06 | p-OH | Br | 45 | 91 | 405 | 540 | 163–165 | 160–166 | (52) | |
| 07 | o-OCH3 | Br | 40 | 80 | 356 | 540 | 87–90 | 87–89 | (52) | |
| 08 | p-CH2Br | Br | 45 | 82 | 365 | 487 | 82–84 | 83–86 | (53) | |
| 09 | H | Cl | 720 | 74 | 329 | 27 | 69–72 | 69–71 | (51) | |
| 10 | p-OH | Cl | 720 | 65 | 289 | 24 | 82–84 | 164–166 | (52) | |
| 11 | o-OCH3 | Cl | 720 | 65 | 289 | 24 | 87–90 | 87–89 | (52) | |
| 12 | p-CH2Br | Cl | 720 | 70 | 312 | 26 | 82–84 | 83–86 | (53) | |
| 13 | H | I | p-CH3 | 35 | 90 | 401 | 687 | 41–44 | 42–44 | (54) |
| 14 | H | I | p-NH2 | 40 | 88 | 392 | 588 | 50–53 | 51 | (55) |
| 15 | H | Br | p-CH3 | 45 | 85 | 378 | 505 | 41–44 | 42–44 | (54) |
| 16 | H | Br | p-NH2 | 50 | 84 | 374 | 449 | 50–53 | 51 | (55) |
Reaction conditions: aryl halides (1 mmol), phenylboronic acids (1.2 mmol), K2CO3 (1.5 mmol), H2O (2 mL), and the Fe3O4@Guanidine-Pd (0.22 mol %) catalyst was agitated at 70 °C for 20–720 min.