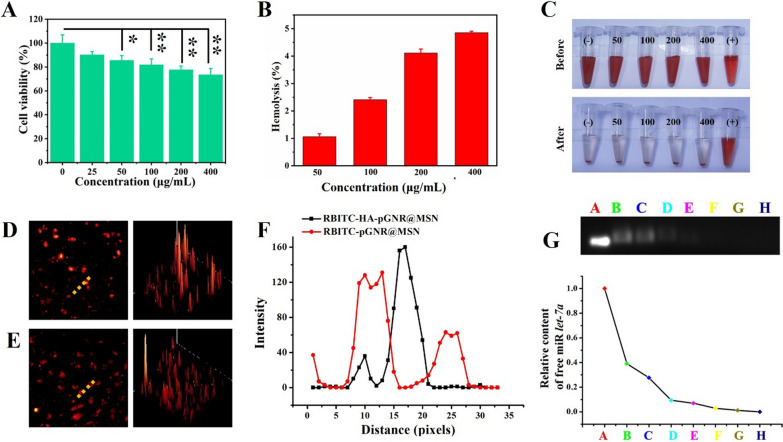
Fig. 3.
Assessment of biosafety, fluorescent properties, and drug loading of the HA-pGNR@MSN nano co-deliverysystem. A Cell vitality after 24 h treatment with HA-pGNR@MSN at various concentrations. B Hemolysis of HA-pGNR@MSN. C Digital photographs after incubation with RBCs at various concentrations for 2 h. D Fluorescent RBITC-labeled HA-pGNR @MSN and E pGNR@MSN. F Corresponding fluorescence profiling graph analyzed with Image-Pro Plus software. G Visualization of gel retardation assay of HA-pGNR@MSN binding with let-7a at different volume HA-pGNR@MSN:let-7a ratios: 0, 40:1, 80:1, 120:1, 160:1, 200:1, 240:1, and 280:1 from A to H, respectively. HA, hyaluronic acid; pGNR, NH2-PEG-COOH-modified gold nanorod; MSN, mesoporous silica nanoparticle; PTX, paclitaxel; let-7a, lethal-7a; RBC, red blood cell; RBITC, Rhodamine B isothiocyanate