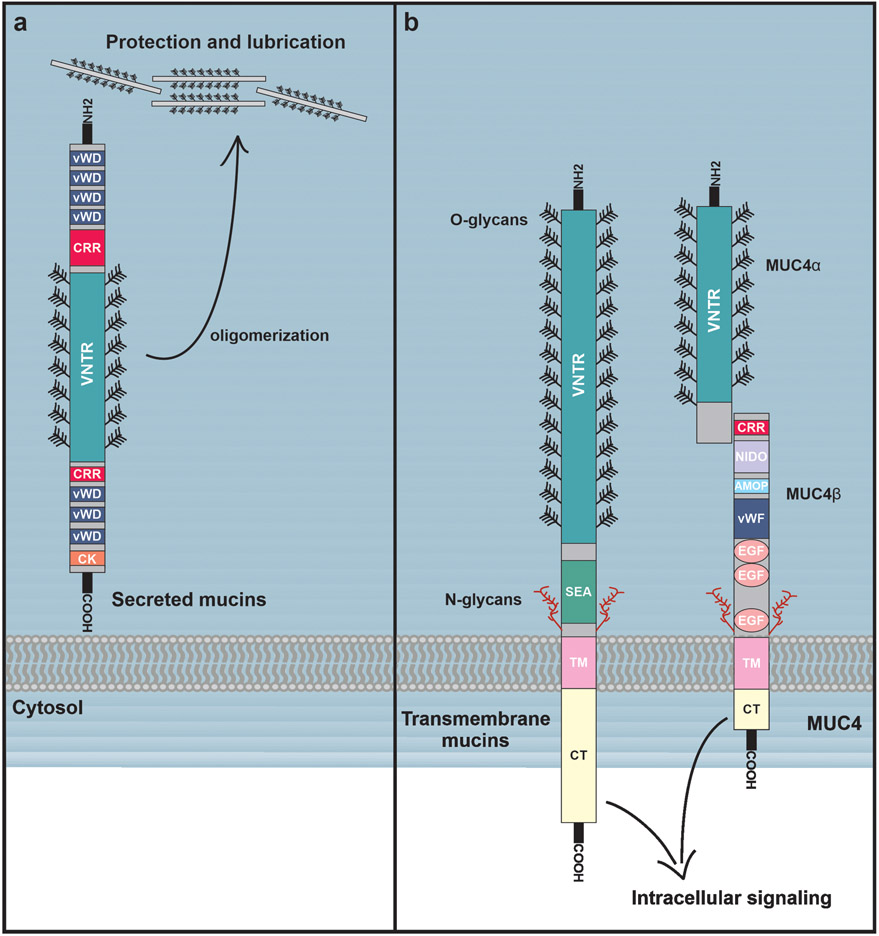
Figure 1.
Structures of secreted and membrane-bound mucins. (A) Secreted mucins contain multiple vWD (von Willebrand factor D) domains, cysteine-rich regions (CRRs), as well as a single C-terminal cysteine-knot (CK) domain. The middle regions of the secreted mucins consist of a highly O-glycosylated VNTR domain which contains cysteine-rich regions dispersed throughout. Secreted mucins oligomerize through their CRR and CK domains, forming large highly viscous aggregates that contribute to the lubrication and protection of epithelial surfaces. (B) Membrane-bound mucins are structurally similar to secreted mucins, and contain vWD domains, CRRs, and O-glycosylated VNTR domains, but are distinguished by a single transmembrane domain that tethers them to the cell surface. Membrane-bound mucins also contain SEA domains as well as some N-glycosylated regions. Notably, in MUC4 the SEA domain is replaced with NIDO and AMOP domains. MUC4 also contains three EGF-like domains, and is proteolytically cleaved to produce noncovalently-linked MUC4ɑ and MUC4β subunits. While membrane-bound mucins are also critical to the protection and lubrication epithelial surfaces, they can simultaneously play extensive roles in cellular signaling, often through their cytoplasmic tails.