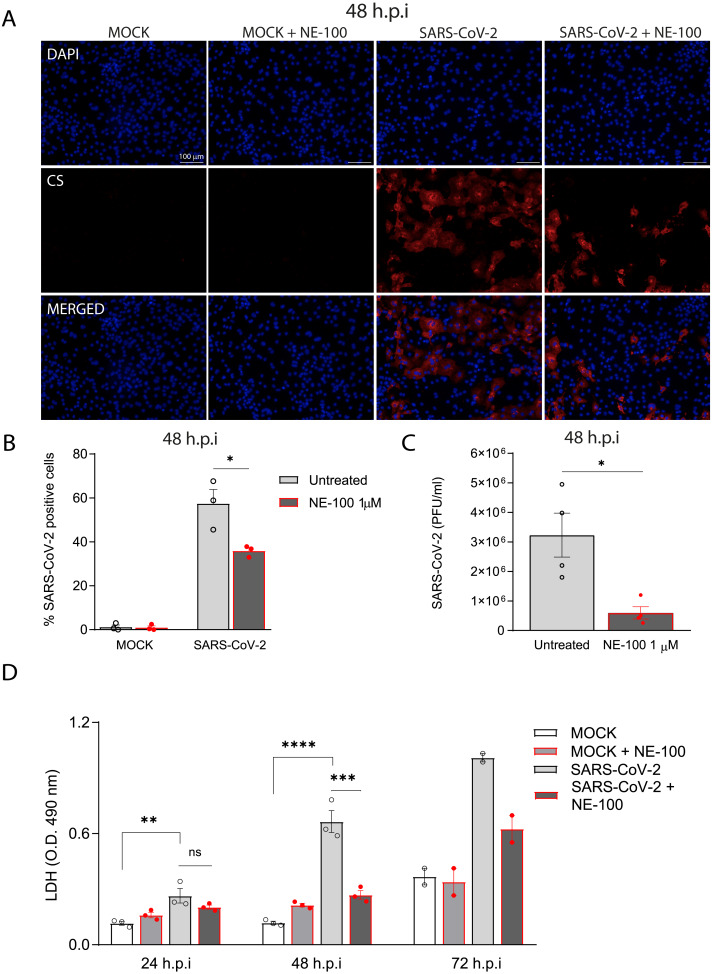
Figure 2. S1R inhibition reduces SARS-CoV-2 infection, replication, and cytotoxicity in hiPSC-CMs.
(A) hiPSC-CMs were pre-treated for 24 h with 1 µM NE-100 and infected with SARS-CoV-2 at multiplicity of infection (MOI) of 0.1. Cells were evaluated at 48 h post-infection (h.p.i). Representative immunostaining images show infected hiPSC-CMs positively stained for convalescent serum (CS) in red and no signal detection in mock conditions (N = 3); scale bar = 100 µm. (B) The percentage of infected hiPSC-CMs was assessed by quantification of CS positive cells in SARS-CoV-2-infected and mock-infected conditions exposed or not to S1R high-affinity antagonist NE-100 (N = 3). (C) Plaque forming units assay for the supernatants of the SARS-CoV-2 infected hiPSC-CMs (N = 4). (D) Cell death was measured in the supernatant by LDH activity at 24, 48 (N = 3) and 72 h.p.i (N = 2). Data are represented as the mean ± S.E.M, analyzed by Nested t-test (p = 0.0003) (B), unpaired two-tailed Welch’s t-test (p = 0.0336) (C) and ordinary one-way ANOVA followed by Holm-Sidak’s post-hoc (24 h p = 0.0089; 48h p < 0.0001 and p = 0.0001) (D). Data points represent independent experiments. * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001 p < 0.0001.