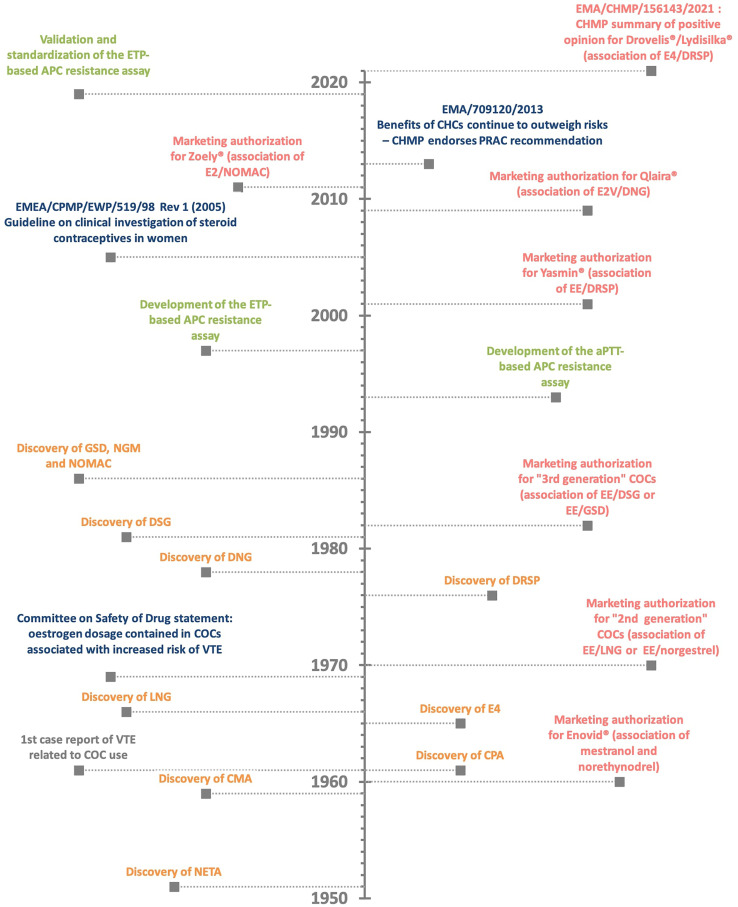
Figure 1.
The history of combined oral contraceptive (COC) and the related risk of venous thromboembolism (VTE). Discovery of estrogen and progestin compound (in orange) - marketing authorization for combined oral contraceptives (in red) – authorities’ statement (in blue) – assay development (in green). APC, activated protein C resistance; aPTT, activated partial thromboplastin time; CHMP, Committee for Medicinal Products for Human Use; CHC, combined hormonal contraceptives; CMA, chlormadinone acetate; COC, combined oral contraceptives; CPA, cyproterone acetate; DNG, dienogest; DRSP, drospirenone; DSG, desogestrel; EE, ethinylestradiol; EMA, European Medicines Agency; ETP, endogenous thrombin potential; E2, 17β-estradiol; E2V, estradiol valerate; E4, estetrol; GSD, gestodene; LNG, levonorgestrel; NETA, norethisterone acetate; NGM, norgestimate; NOMAC, nomegestrol acetate; PRAC, Pharmacovigilance Risk Assessment Committee; VTE, venous thromboembolism event.