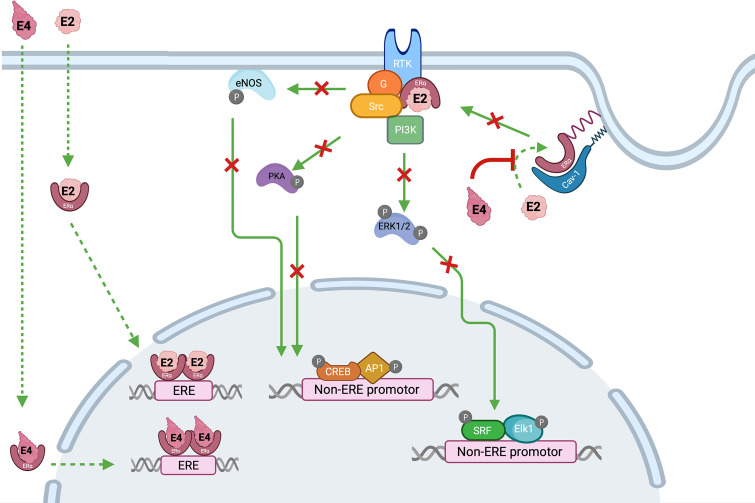
Figure 6.
Interaction between estrogens, i.e., estradiol (E2) and estetrol (E4) and estrogen receptors alpha (ERα). Estradiol activates both membrane and nuclear actions of ERα while E4 is an agonist of nuclear activity but an antagonist of the ERα-dependent MISS pathway. Nuclear activity is the result of an interaction between estrogens, i.e., E2 or E4, and the ERα located in the cytoplasm. This binding leads to the dimerization and the translocation of the complex into the nucleus, where it interacts with ERE DNA sequences in target genes. The ERα-dependent MISS pathway consists of a rapid nongenomic activity playing an important role in the endothelial effect of estrogens. Palmitoylation of ERα allows them to anchor to the plasma membrane caveolae where they associate with caveolin-1 (Cav-1). Upon E2 stimulation, ERα is de-palmitoylated and dissociated from Cav-1, to interact with protein kinase (Src and PI3K), G-coupled protein ai (Gai) leading to signaling cascade (Akt, Pka, ERK1/2) and endothelial NO synthase activation. On the other hand, E4 is devoid of ERα MISS activity and even, is also able to antagonize E2-induced MISS effect, especially in the endothelium. AP1, activator protein 1; Cav-1, caveolin 1; CREB, cAMP-response element-binding; Elk1, ETS like-1 protein; ERK, extracellular signal-regulated kinases; eNOS, endothelial nitric oxide synthase; ER, estrogen receptor; ERE, estrogen responsive element; E2, estradiol; E4, estetrol; G, G protein (sub-unit ai); MISS, membrane-initiated steroid signaling; PI3K, phosphoinositide 3-kinase; RTK, receptor tyrosine kinase; SRF, serum response factor.