Table 4.
Comparison estradiol (E2), ethinylestradiol (EE) and estetrol (E4).
| Estradiol | Ethinylestradiol | Estetrol | ||
|---|---|---|---|---|
| Origin | Natural Synthetized in the growing ovarian follicle, corpus luteum, placendal, adrenal and Leydig cells, liver, endometrium, brain muscle and fat tissue |
Synthetic derivative | Natural Exclusively synthetized in the fetal liver |
|
| Chemical structure |
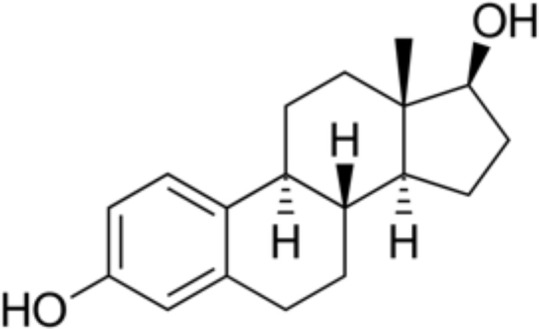
|
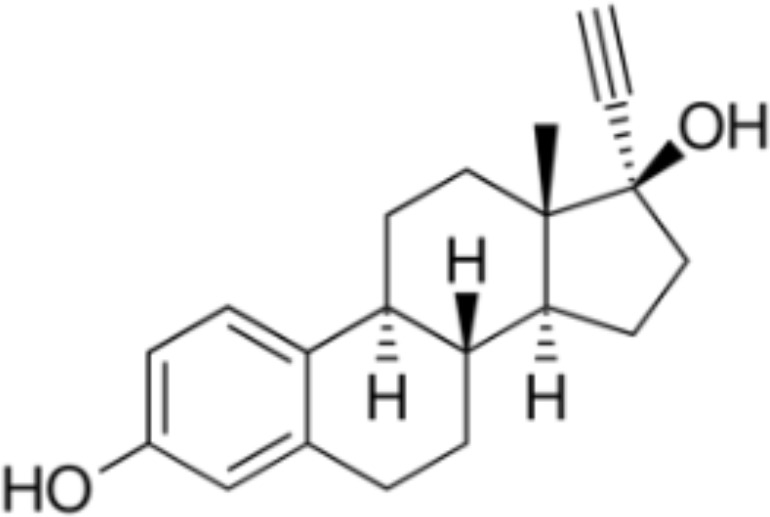
|
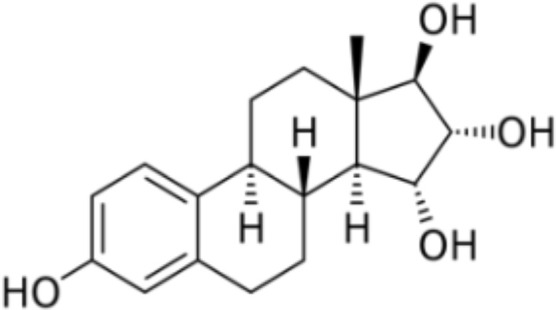
|
|
| Dosage in COC | 20-50 µg | 1-3 mg | 15 mg | |
| Associated progestogen in COC | NOMAC, DNG | LNG, NETA, NGM, DSG, GSD, DRSP, CPA, DNG, CMA | DRSP | |
|
PK
profile |
Oral bioavailability | Low oral bioavailability but administered either micronized or esterified. | Good oral bioavailability | Good oral bioavailability |
| Metabolism | High metabolism into E1 (sulfate) and E3 (sulfate) | High metabolism into various conjugates (glucuronidated, sulfated and hydroxylated metabolites) | No metabolization | |
| Half-life time | Half-life time around 35 hours (E1 serves as precursor of E2 and can be transformed back into E2) | Half-life time around 12 hours | Half-life time around 28 hours | |
| PD profile | High selectivity for ER (higher affinity for ERα) | High selectivity for ER (higher affinity for ERα); First Native Estrogen with Specific action in Tissues (NEST) | ||
| Impact on liver protein synthesis | Minor (non negligeable contribution of E1) | Major | Minor | |
COC, combined oral contraceptives; CMA, chlormadinone acetate; CPA, cyproterone acetate; DNG, dienogest; DRSP, drospirenone; DSG, desogestrel; ER, estrogen receptor; E1, estrone; E2, estradiol; E3, estriol; GSD, gestodene; LNG, levonorgestrel; NETA, norethisterone acetate; NGM, norgestimate; NOMAC, nomegestrol acetate; PD, pharmacodynamic; PK, pharmacokinetic.
