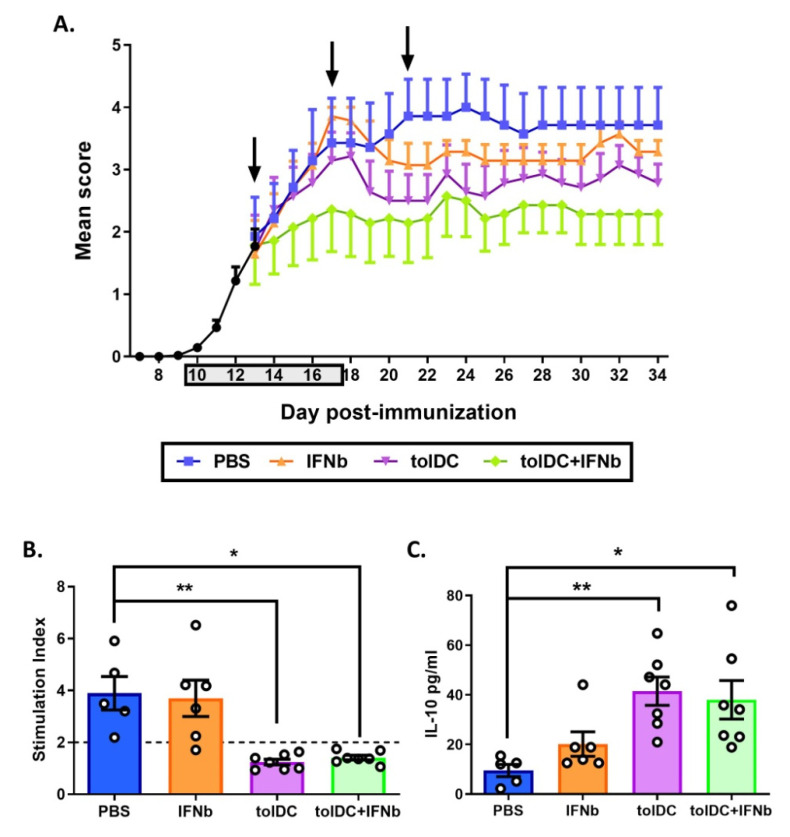
Figure 6.
Combined therapy of antigen-specific VitD3-tolDC-MOG+IFN-beta ameliorates clinical signs of EAE. (A) Representation of daily mean clinical score of mice treated with vehicle (PBS) (blue), IFN-beta (IFNb) [5000IU] (orange), VitD3-tolDC-MOG [1 × 106 cells] (tolDC) (violet) or VitD3-tolDC-MOG+IFN-beta (tolDC+IFNb) (green) (n = 7/group) for 34 days of follow-up. Gray square in the X axis and arrows indicate daily treatment period with IFN-beta (from day 10 to 17 post-immunization, pi) and VitD3-tolDC-MOG administration (days 13, 17 and 21 pi), respectively. (B) Analysis of antigen-specific T cell reactivity to MOG35-55 in splenocytes from mice treated with vehicle (PBS), IFN-beta (IFNb), VitD3-tolDC-MOG (tolDC) or VitD3-tolDC-MOG+IFN-beta (tolDC+IFNb) on day 34 pi. (C) Level of IL-10 in the supernatant of re-stimulated splenocytes with MOG35-55 antigen from each group of mice. Errors bars correspond to SEM. * p < 0.05, ** p < 0.01.