Abstract
Zika virus (ZIKV) was first discovered in 1947 in Uganda. ZIKV did not entice much attention until Brazil hosted the 2016 Summer Olympics Game, where ZIKV attracted a global audience. ZIKV is a flavivirus that can be transmitted chiefly through the biting of the mosquito or sexually or by breastfeeding at a lower scale. As time passed, the recent discovery of how the ZIKV causes congenital neurodevelopmental defects, including microcephaly, makes us reevaluate the importance of ZIKV interaction with centrosome organization because centrosome plays an important role in cell division. When the ZIKV disrupts centrosome organization and mitotic abnormalities, this will alter neural progenitor differentiation. Altering the neural progenitor differentiation will lead to cell cycle arrest, increase apoptosis, and inhibit the neural progenitor cell differentiation, as this can lead to abnormalities in neural cell development resulting in microcephaly. Understanding the importance of ZIKV infection throughout the years, this review article gives an overview of the history, transmission routes, pathogenesis, animal models, and diagnosis.
Keywords: Zika virus (ZIKV) centrosome, flavivirus, microcephaly
BACKGROUND
Zika Virus (ZIKV), along with yellow fever (YF), Japanese encephalitis (JE), West Nile (WN), and the dengue (DEN) viruses, is characterized as a positive-sense single-stranded RNA virus and belongs to the family of Flaviviridae and the genus flavivirus. Although these viruses are only a few listed here, there is an in-depth summary of the family Flaviviridae, which contains many other viruses, in a 2010 review (1). ZIKV can be classified into distinct African and Asian lineages through phylogenetic analysis as both lines emerged approximately from East Africa during the late 1800s or early 1900s (2). The Asian line originated during the virus’s migration from Africa to Southeast Asia, in which it was first detected in Malaysia (2). ZIKV is a mosquito-borne flavivirus typically causing mild illness that has rapidly expanded its scope worldwide, causing maternal infection and viral transmission to the fetus (3). ZIKV’s impact on the health of newborns is vital in understanding how viral infections affect cellular physiology (4). ZIKV is associated with microcephaly of newborns whose mothers were infected with ZIKV. Viral microcephaly is a congenital disability where a baby’s head is smaller than normal compared to babies of the same sex and age (5). Babies with microcephaly often have smaller brains that hinder their development compared to their peers, thus resulting in a range of health problems (5).
The mechanisms of how ZIKV causes microcephaly has been investigated in detail. Most experimental results support the hypothesis that ZIKV infect neural progenitor cells (NPCs) of the fetal brain and result in microcephaly (6). Animal models showed that ZIKV infection caused cell cycle arrest and apoptosis of NPCs and massive neuronal death and axonal rarefaction (7–9). The human neural organoid model also showed that ZIKV infection leads to increased cell death and reduced proliferation, resulting in decreased neuronal cell-layer volume resembling microcephaly although the mechanisms of the biological effects could be different (10–12). Our studies demonstrated that ZIKV impacts on the centrosome to affect the newborn’s development because the centrosome plays a vital role in cell division (13). Therefore, ZIKV employs multiple mechanisms to result in defects of fetal brain development and cause microcephaly. However, less than 5% of the congenitally infected newborns develop microcephaly and other more than 95% of congenitally infected cases are normal at birth (4, 14, 15). Therefore, the factors that are involved in ZIKV-associated microcephaly remain to be revealed. This review will prospectively summarize five aspects of ZIKV: transmission, origin, pathogenesis, animal models and diagnosis. The future studies of ZIKV are also discussed.
TRANSMISSION OF ZIKV
Mosquito biting
ZIKV is closely related to the Spondweni virus per the phylogenetic analysis, and both viruses are mosquito-borne viruses capable of causing human disease (16). Arboviruses (arthropod- borne viruses) is a general term used to describe hundreds of RNA viruses relying on arthropods such as mosquitoes or ticks to start transmitting the infections to humans by the bite of infected arthropods (insects) (17). Like many other flaviviruses, one of the ways ZIKV is transmitted in humans is through the biting of an infected mosquito, mainly the Aedes aegypti, predominately of the Aedes (Stegomyia) genus (18–20). The Aedes aegypti mosquito usually bites during the day, peaking during early morning and late afternoon/evening in the tropical and subtropical regions (18–20). In looking further into ZIKV transmission through infected mosquitoes, ZIKV has been isolated in the two known ZIKV lineages country of Africa and Asia from several different mosquito species in the Aedes genus (e.g., A. aegypti, A. africanus, A. albopictus, A. apicoargenteus, A. furcifer, A. luteocephalus, A. opok, and A. vittatus) that can potentially act as vectors for viral transmission in those endemic areas (21–30).
Aedes aegypti primarily transmits four viruses that have had the greatest impact on human health, including yellow fever virus (YFV), dengue fever (DENV), chikungunya, and ZIKV. As shown in Figure 1, mosquitos take 5–8 days to develop from eggs via larva to pupa and another 3 days to become adults (31). If the adult mosquito bites a ZIKV-carrying primate, the mosquito likely obtains the virus from the animal blood. Then ZIKV will infect and replicate in the epithelial cells of the mosquito and circulate in its blood and release to saliva tissue. The infectious mosquitos transmit ZIKV to other non-human primates or to humans. The transmitter mosquitos will expand the infected populations to cause outbreaks or epidemics. During the epidemics, ZIKV transmission may occur via sexual action, breastmilk feeding and vertical transmission at a lower scale.
Figure 1.
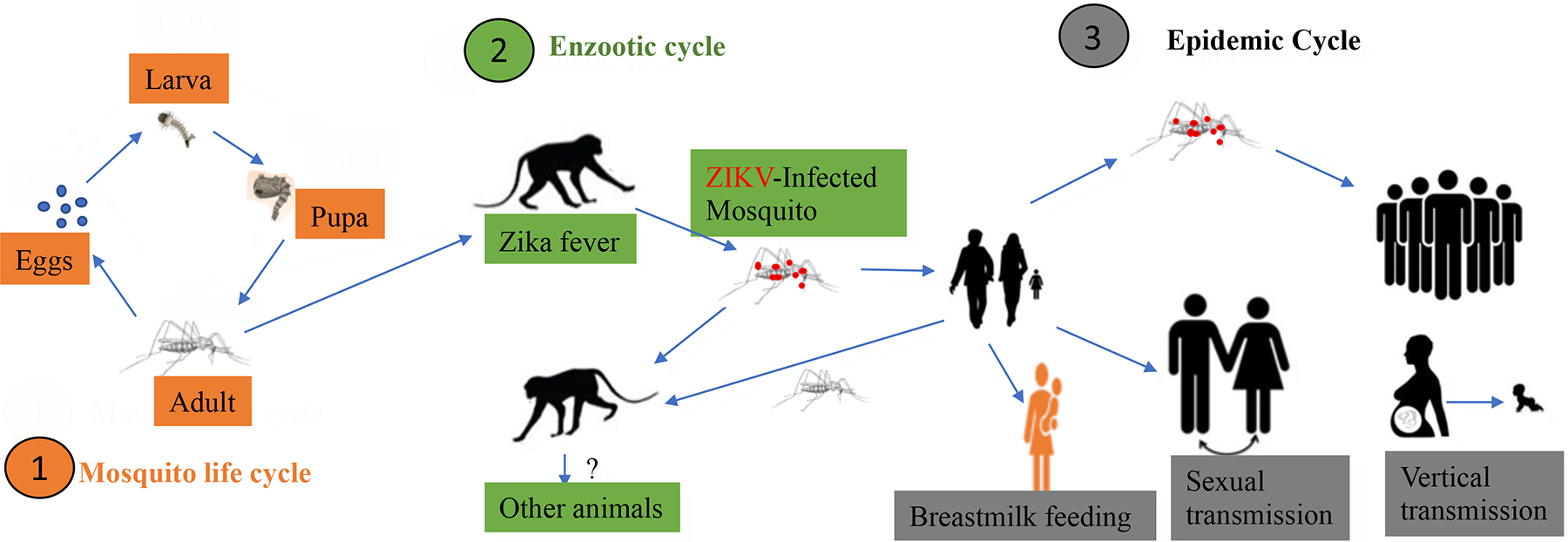
A. The life cycle of a mosquito. B. ZIKV infection and transmission from NHP to NHP or humans. C. Different ways of ZIKV transmission during epidemic.
Sexual transmission
The first reported case of sexually transmitted ZIKV infection occurred in Colorado, USA, in 2008 (32). A female patient who did not have any past travel history to any endemic region experienced ZIKV liked symptoms (32). The husband of the female patient is an American scientist who contracted ZIKV infections as wild Aedes spp. mosquitoes often bit him in the evenings as he routinely performed a mosquito-sampling project in surrounding villages in Senegal in 2008 (33). As soon as the infected American scientist returned home from Senegal, they had vaginal sexual intercourse, which resulted in the direct contact as a transmission route resulting in a likely sexually transmitted infection (33). Since the reporting, there have been many more increased sexually acquired ZIKV cases in non-endemic regions. As their infected partners arrive from endemic areas, they have sexual intercourse, resulting in the increase of ZIKV cases (34). Hence public health indicates the sexual transmission may arise due to the quick spread of ZIKV, which will expand to various geographical regions and to healthy people in this world, not excluding how the effect will impact fertility and the safety of the sperm and ovum banks (35). Therefore, a plan was put into place as a sexual transmission framework was brought upon in 2017 by the World Health Organization (WHO) to understand more about the sexual transmission of ZIKV and how to combat ZIKV epidemic (36). Notably, ZIKV infects testis and was detected from seminal fluid, and, to our knowledge, sexual transmission can be from male to female (32). Interestingly, sexual transmission has never been observed in any other mosquito-borne flavivirus.
Breastfeeding
There is an establishment of ZIKV transmission from mother to child that could cause harm to the child during her pregnancy or the time of giving birth; however, less is known if there is a transmission through breast milk as there is limited data and publications available (37). According to the Centers for Disease Control and Prevention (38, 39), breastfeeding is an investment in health as breastfeeding in general helps lower disease risks for infants and mothers. Due to the limited data available regarding whether there is a potential risk that the human milk can be a vector for ZIKV transmission, the World Health Organization (WHO) made a collective decision that the benefits of breastfeeding for the infant and mother will outweigh the potential risk of ZIKV transmission. This has been also demonstrated by an animal study that demonstrated that suckling mice can be infected with ZIKV through suckling the ZIKV-containing human milk, but human breast milk has potential antiviral activity (40). Even if there could be ZIKV transmission through the mother breast milk, WHO still recommended that mothers who think they are positive or have confirmed cases of ZIKV infection should continue to breastfeed their children (41, 42). Figure 1 shows the vertical transmission, sexual transmission and the breastfeeding transmission.
ORIGIN OF ZIKV LINEAGES
African lineage of ZIKV
ZIKV was first identified in 1947 in the Zika forest in Uganda by British scientists working in the Yellow Fever Research Laboratory, where they isolated ZIKV from a sentinel rhesus monkey (16, 21). Dick et al (21), on April 18, 1947, led a group of scientists and had put a rhesus monkey in a cage on a tree platform in the Zika Forest that developed a fever. The monkey was a species of Rhesus, and the number given to the rhesus monkey was 766 by the Rockefeller Foundation’s program for research on jungle yellow fever (43). As two days went on, Rhesus 766 was still showing fever symptoms, so Rhesus 766 was brought into the Foundation’s laboratory in Entebbe, Uganda and its serum was collected and injected into the brain of mice (43). As time passed, 10 days later, all mice that were introduced with the serum into the cerebrum were sick. Then, Dick et al isolated the virus from the mouse brain and named it as ZIKV, and the strain was MR766 (43). Thus, the first isolated African strain of ZIKV was named MR766 (44). The British scientists were looking into jungle yellow fever as they stumbled onto the finding of ZIKV African lineage as they brought it to fruition.
The first known isolation of ZIKV from humans was reported in 1954 (16). A 10-year old Nigerian girl came into a rural dispensary and complained about fever and headache (16). As scientists were investigating in the Afikpo Division, Eastern Nigeria, an outbreak of jaundice that they suspected of being yellow fever, they wanted to know if that patient had yellow fever (16). So, the patient’s serum was injected into adult Swiss mice bred, and a couple of days later, ZIKV was subsequently isolated from the mice (16). There were two other cases of ZIKV infection reported in Nigeria in 1954, as case one consisted of a 30-year old Nigerian male and case two consisted of a 24-year old Nigerian male as both cases were confirmed to have ZIKV by the rise in serum neutralizing antibodies (16). In summary, the results of these investigations into yellow fever contributed to the finding of ZIKV as ZIKV is an arbovirus, which the mosquitoes can transmit to infect at the very least monkeys and humans.
Asian lineage of ZIKV
Outside of Africa, the first prototype strain of the Asian lineage of ZIKV was isolated in 1966 from mosquito (Aedes aegypti) pools collected in Bentong, Malaysia (29). The sequence analysis detected this Asian lineage of ZIKV as a distinct lineage of the origin of ZIKV, which the Asian lineage of ZIKV is considered to be a part of the African lineage of ZIKV (45). In Asia between 1954 and 1976, there were no recordings of human clinical ZIKV cases when using serological tests or virus isolation procedures (46). ZIKV cases likely remained undetected because ZIKV disease was primarily an illness that stays below the surface of clinical detection (46). Also, there was not a routine checkup to perform diagnostic tests for ZIKV (46).
As 11 years passed, a serological study reported that ZIKV outbreak in Asia occurred in central Java, Indonesia in 1977 (47). Reports of ZIKV infection started to happen in the latter half of the rainy season in central Java, Indonesia in 1977 when Aedes aegypti usually flourishes. Seven patients were hospitalized in Tegalyoso Hospital, Klaten, Central Java, Indonesia as each one of them had a high fever, malaise, stomach ache, dizziness, and anorexia (47). These seven patients were later tested, and the data collected from these seven ZIKV cases showed the clinical characteristics exhibited by the patient who endures ZIKV to be usually mild and non-fatal (47). ZIKV is highly likely transmitted by Aedes aegypti, which is a known possible vector in Malaysia (29).
ZIKV African lineage and ZIKV Asian lineage are both related and distinguishable by detailed genetic analysis of the genomic RNA sequence (45). When comparing strains, the primary variability is the differences in how many available potential glycosylation sites (17). The increase in the distribution area of the ZIKV made its way to French Polynesia (FP) as Zika fever is an emerging infectious disease (48). As of October 2013, ZIKV confirmation in FP reached a total of 8,700 suspected and 400 laboratory-confirmed cases (48). The complete coding sequence of the virus was obtained from a 51-year old woman in November 2013 (48). As she was returning from French Polynesia (FP), she was hospitalized in metropolitan France as she was feeling ill with fever, headache, myalgia, arthralgia, and a rash (48). The coding sequence revealed that the patient was infected with a ZIKV Asian lineage, with about 99.9% nucleotide and amino acid homology with separation that went around during the 2000s in Southern Asia and the Pacific Islands (17). These finding points in the direction of the second lineage of ZIKV, which has been suggested that the origin of ZIKV lineage from Uganda probably spread to Malaysia around 1945 and from there, the virus continues spreading to reach Micronesia around 1960, forming the Asian cluster (45, 49). Therefore, there are two known lineages of ZIKV today, an African lineage and an Asian lineage (50). The African lineage is ancestral to the Asian lineage as both share a similar amino acid sequence; thus, there are two known lineages of ZIKV.
The data collected onto the world map suggests that distinct ways of ZIKV transmission among humans, animals, and mosquitoes have occurred throughout tropical and subtropical regions of Africa and Asia for more than 70 years. The timeline of events in ZIKV history is summarized below in Figure 2.
Figure 2.

World map showing the timeline of events of Zika virus. As Zika virus travels from one country to another, the world map is labeled with numbers, years, and colors to break down how Zika virus started and evolved over the years.
PATHOGENESIS OF ZIKV
ZIKV infection of permissive cells
There is not much information about ZIKV pathogenesis as researchers are still conducting research; however, understanding the life cycle of ZIKV development will give us a better understanding of ZIKV pathogenesis. As mentioned earlier in the section on ZIKV transmission through an infected mosquito bite, the primary mosquito is the Aedes aegypti, predominately of the Aedes (Stegomyia) genus. The Aedes mosquitos will lay their eggs in places that contain high moisture which the eggs will grow from larvae to pupae and finally become an adult [Figure 1 and (51)]. The duration for this to happen comes about one-and-a-half to approximately three weeks for the cycle to be completed (51). A female adult mosquito can produce a massive quantity of eggs per batch on an average of 100 to 200 eggs when they endure a blood meal as they average five batches of eggs in their lifetime (51). The minimum number of eggs they can produce in their lifetime is 500 eggs and their maximum eggs production in their lifetime is 1000 eggs. The adult female mosquito will usually bite humans and animals to get their blood to develop her eggs. As the Sylvatic cycle transitions into the Urban cycle, that will affect humans as the Aedes mosquitos will require biting a human for their blood (51).
The infected Aedes mosquito with ZIKV pierces through the human skin as the infected mosquito saliva lubricates the skin in which the saliva and human blood come in direct contact. The surface of ZIKV has Envelop (E) proteins that are primarily involved in attaching to the host cell membrane as the virus is internalized by endocytosis (51). As shown in the Figure 3, the attachment depends on the interaction between viral E protein and receptor. AXL, Tyro3, TIM1 and DC-sign have been identified to be the receptors for ZIKV to infect its permissive cells (52, 53). But the conclusive statement still needs further investigation because recent results have been disputed (54, 55). Then, viral entry is completed by endocytosis via endosomes. As the capsid breaks apart in the cytoplasm, there is a release of the viral RNA into endoplasmic reticulum (51). The positive-sense RNA genome will be translated by the host ribosomes attached to the endoplasmic reticulum, which results in a polyprotein processed by proteolytic activity for the structural and non-structural proteins (51). When all parts are assembled correctly, the virion will be transported out of the cell by endosomal sorting complexes through ERGIC (endoplasmic reticulum-Golgi intermediate compartment) to the Golgi apparatus (51). As exocytosis is used as a mature virion exits the cell in which a single ZIKV can rapidly increase the amount of the virus as the virus will take over the host immune defense system (51).
Figure 3.
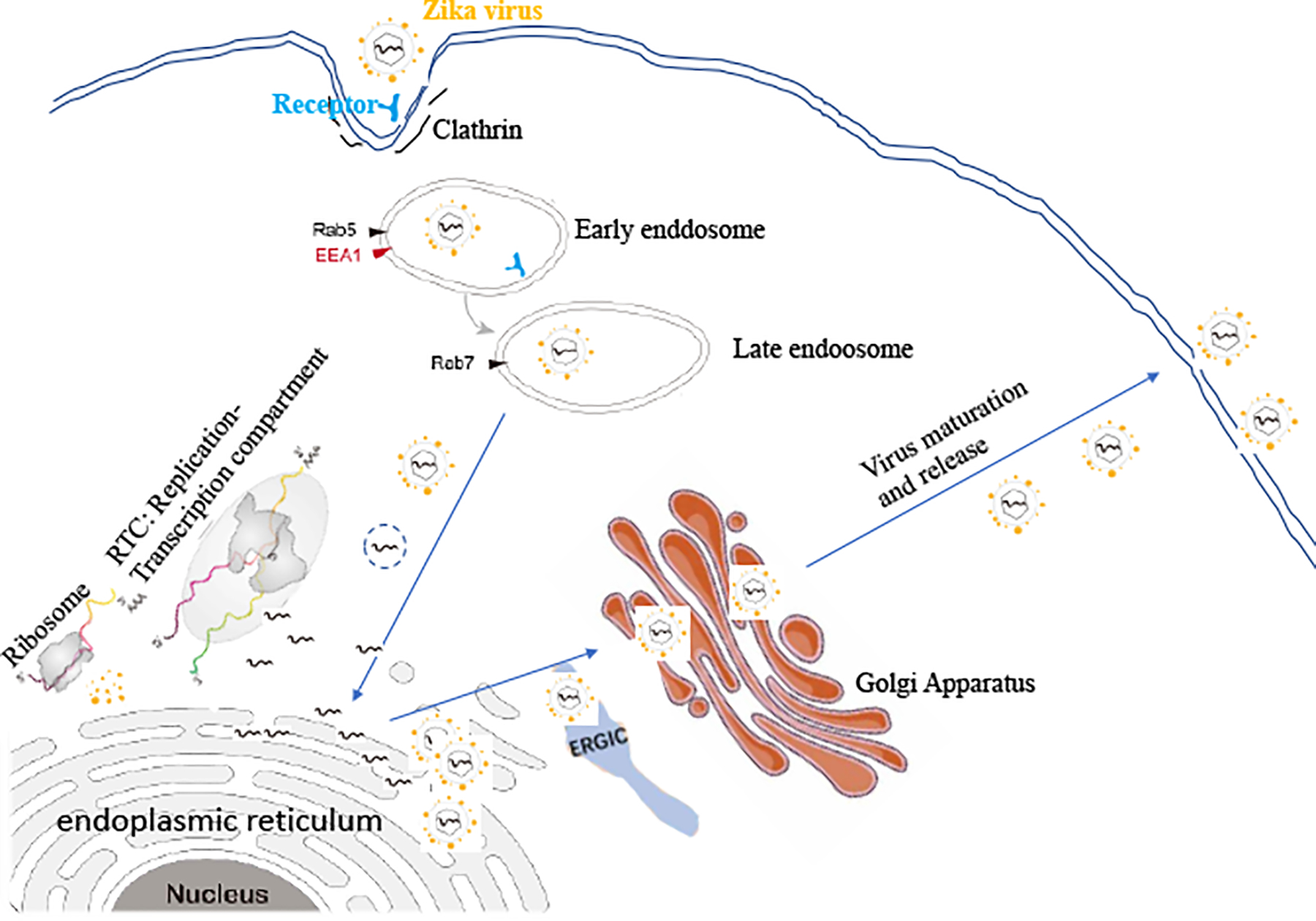
The virus life cycle in the host cells.
ZIKV and microcephaly
For microcephaly in neonates in September 2015, there were multiple reports from researchers about the increase in numbers of neonatal microcephaly cases among pregnant women giving birth to their kids in northeastern Brazil (56, 57). Afterward, there was an increase in neonatal microcephaly cases reported in southeast Brazil (58, 59). Isolation of ZIKV has been retrieved from the amniotic fluid of pregnant mothers with infants that had confirmed microcephaly (56, 58–60). The isolation of ZIKV has also been retrieved from the brain of a fetus with congenital central nervous system abnormalities (4). In Brazil, a systematic study linked ZIKV to microcephaly, as the investigators followed patients in Rio de Janeiro, Brazil to report on the clinical manifestations of the acute ZIKV infection in mothers and how that affected the fetuses (61). Pregnant women were enrolled in the study who had a rash that developed within the previous 5 days. The pregnant women’s blood and urine were obtained to test for ZIKV by utilizing reverse transcriptase (RT)- polymerase chain reaction (PCR) assays. They followed the women prospectively to collect the data on the pregnancy and infants’ outcomes. They enrolled a total of 345 women from September 2015 through May 2016 where 182 women (53%) tested positive for ZIKV PCR in the blood, urine, or both. The timing of acute ZIKV infection fell in a range from 6 to 39 weeks of gestation. In the ultrasound studies there were a total of 153 women as 59 women tested positive for ZIKV during pregnancy. The remaining 75 women who are positive with ZIKV declined to analyze their imaging as either one the facility was too far to commute to or because they fear knowing the truth if their child has fetal abnormalities related to ZIKV infection. For the women who were negative for ZIKV infection, they still receive regular prenatal care. In the infant’s outcome, there was a total of 49 out of the 117 live born infants (42%) that got ZIKV in the utero as the infants possess abnormal findings during the first month of their life. As they analyzed the abnormalities, they found the central nervous system (CNS) was affected the most. Microcephaly was seen in infants whose mothers were infected with ZIKV in weeks 8, 12, 30, and 38 of gestation. Although there is only a mild clinical symptom in the mother having ZIKV infection during pregnancy, that should not be taken lightly as ZIKV will harm the fetus which can come in the form of fetal death, fetal growth restriction, and a whole lot of abnormalities in the central nervous system (61).
A review by Wen et al not only listed the approaches used during 2016–2017 to investigate the ZIKV-caused microcephaly but also summarized the mechanistic information of how ZIKV caused microcephaly (62). In terms of the models, the approaches include 1) two dimensional (2D) cells culture of NPCs (63); 2) 3D NPCs; 3) mouse models, and 4) NHP (non-human primate) models. First, the 2D studies using human NPCs revealed that ZIKV infection dysregulated cellular gene expression, mitigated NPC growth, induced autophagy or apoptosis and increased cell death (63–66). Secondly, studies using 3D models found that ZIKV decreased neuronal cell layer thickness and overall volume, attenuated organoid growth, and impaired cortical growth and folding (12, 64, 67). Moreover, the IFN deficient mouse models (A129 or AG129) showed that ZIKV was detected in placenta and brain and caused transmission to fetus, resulting in central neural system (CNS) neurodegeneration (68–71). Certainly, non-human primate is the best model for ZIKV infection and pathogenesis. ZIKV infection in Rhesus macaques caused symptoms like in humans and viral particles were detected in saliva, urine and CSF (cerebrospinal fluid). Many studies using the in vitro and in vivo models demonstrated that ZIKV has direct association with microcephaly.
Given how ZIKV can impact the health of newborns before they even have a chance of living a normal healthy life, it is crucial to continue to understand how the viral infection affects cellular physiology (4). It is believed microcephaly could have a relation to the abnormal function of centrosomes (17). Centrosomes are cellular organelles that serve as the primary microtubule-organizing centers of an animal cell. Centrosomes have a role in mitosis and vesicles migration, polarization, and trafficking (17). Studies done before had proven that in vitro ZIKV infection alters cellular functions which include mitosis along with cells that were infected expressing spindle misorientation and increased centrosome numbers (66, 72–74). An increased number of centrosomes correlates with microcephaly. A delay in mitosis and an increase in apoptosis can happen when there is an increase in centrosome number (17). As cells that are infected with the ZIKV have disrupted centrosome organization and mitotic abnormalities, thus leading to altered neural progenitor differentiation (66, 72–75). Our recent studies also demonstrated that ZIKV infection disrupted the structure and function of pericentral centromere materials (PCM) (13). Therefore, when centrosome and neural progenitor differentiation is afected by ZIKV, this could lead to the result of the development of microcephaly as neural progenitor differentiation will go to cell cycle arrest which will increase apoptosis and stop the neural progenitor cell differentiation resulting in abnormalities in neural cell development.
ZIKV and Guillain-Barré syndrome (GBS)
The characteristics of ZIKV can expand its scope from microcephaly in neonates to GBS in adults (76, 77). Although there have been reports of the link between ZIKV and GBS, many sources say more research is needed. During the ZIKV outbreak in French Polynesia, an unexpectedly high number of GBS cases was observed. In December 2013, the first patient who presented a “Zika-like syndrome” symptoms including low fever, myalgia, rash, and conjunctivitis was diagnosed as GBS and hospitalized (78). The ZIKV-associated GBS patients are mostly male and exhibit neurological symptoms. During the epidemic of ZIKV in Brazil, cases of GBS increased by 20 folds. Collectively, these epidemiological data reinforce the hypothesis of a relationship between ZIKV and GBS. Ocular complications in adults have been reported (79). The Centers for Disease Control and Prevention is actively continuing to investigate the association between ZIKV and GBS. Taken together, ZIKV is known to cause congenital neurodevelopmental defects, including microcephaly in kids and the possibility of GBS in adults.
Mechanistic studies of ZIKV Pathogenesis in CNS
To understand the mechanisms of how ZIKV causes microcephaly and other CNS disorders, multiple models have been applied. As mentioned above, ZIKV-associated disorders mostly occur to the congenitally infected neonates as shown in Figure 4A. The in vitro studying models include 2D cell cultures and 3D cell cultures. And the animal models are primarily mouse models and NPH models. Primary human NPCs (hNPCs) (Figure 4B), hiNSC-derived NPCs, microglial cell lines and human dermal fibroblast cells have been used for investigating the effects of ZIKV on cellular function, growth, death, apoptosis and innate defenses (Figure 4C). 3D NPCs such as hiPSC-derived cerebral organoid, hiPSC-derived forebrain organoid and hiPSC-derived cerebral neutrospheres were used to study the viral effects on neural cell differentiation. First, it was found that ZIKV has a wide range of permissive cell types (80) supporting ZIKV’s efficient infection and replication. ZIKV infection causes increased cell death, disrupted cell cycle progression, dysregulated gene expression, attenuated hNPC growth, and apoptosis (55, 63–66, 81–83). ZIKV replicates in primary human neural progenitors and induces cell death, inhibition of the Akt–mTOR pathway, activation of autophagy, centrosomal depletion and mitochondrial sequestration of phospho-TBK1 (83, 84). ZIKV preferentially infects NSCs, astrocytes, oligodendrocyte precursor cells, and microglia (85). ZIKV infects NPCs in the VZ, impairs cell cycle progression, activates immune responses (86), induces antiviral gene expression (86). AXL mediates ZIKV entry and modulates innate immune responses (52, 87). ZIKV can infect and replicate primary human trophoblasts (PHTs) (88, 89); PHT cells resist ZIKV infection and release IFNλ1 (89, 90). ZIKV infects and replicates in Hofbauer cells and induces IFN, proinflammatory cytokines, and antiviral gene expression (89). ZIKV infects cells via AXL and other factors and induces the transcription of TLR3, RIG-I, and MDA5. Increased cell death, reduced proliferation, decreased neuronal cell layer thickness and overall size were seen in ZIKV-infected 3D culture models (10–12, 64, 74).
Figure 4.

In vitro and in vivo models for investigating ZIKV-associated CNS disorders and outcomes.
Animal models for ZIKV study started from using immunocompromised mice like A129 or AG129 that lacks IFNI or IFNII responses (Figure 4B). Those studies revealed how ZIKV is transmitted from mother to fetus. Although the wild type (WT) adult mouse is important for ZIKV study but it is resistant to ZIKV infection, (7, 91, 92). The mice used as models for ZIKV studies so far have deficiencies in either the IFN or the IFN receptor which are caused either genetically or via antibody treatment (93). The A129 mouse has genetically knocked out its receptors for type I IFN (IFN alpha and beta) and hence not susceptible to stimulation of IFN-alpha and -beta, and is vulnerable to ZIKV infection (70). The AG129 mouse lacks the IFN-alpha, -beta, and -gamma receptors (70, 94). Another strain of mouse called triple knockout, with knockout of three genes (irf3−/−, irf5−/− and irf7−/−), produces little IFN-alpha and -beta, and is also fatally susceptible to ZIKV infection (94). The importance of IFN-alpha/beta signaling in preventing ZIKV infection has also been confirmed using anti-IFN-alpha receptor antibody. The blockade of the type I IFN response using the anti-IFNaR1 antibody in WT pregnant mice enabled ZIKV to cross the placenta to infect the fetus (95). Besides IFN-alpha, -beta, and -gamma receptors, it has been recently demonstrated that fetus lacking IFN-λ receptor had increased ZIKV replication in the placenta and fetus (92). With these mouse models, it was found that ZIKV reduced proliferative zones, disrupted cortical layers, interfered with the differentiation of NPCs, impaired neurogenesis and folding. Other animal model is using the NHP like Rhesus macaques to confirm the information obtained from mouse models. It was reported that ZIKV was detected in the brain and caused signs of neurologic disease in NHPs. In NHPs ZIKV presents in saliva, urine, and cerebrospinal fluid and causes human-like symptoms (96–98). The effects of ZIKV infection on cells and animals are encapsulated in Figure 4C.
ANIMAL MODELS OF ZIKV STUDIES
ZIKV has continued to spread worldwide for many years, but ZIKV pathogenesis and mechanism are not fully understood yet as scientists continue to do more research. Using animal models of infection and disease is necessary to be the starting point of understanding how ZIKV pathogenesis and mechanism develop in order to develop a vaccine (99). Animal models are a crucial part of understanding ZIKV pathogenesis and mechanism to be able to develop a vaccine.
Small animal models
Immunocompetent mouse models
Animal models of ZIKV infection did not gain enough traction until the recent ZIKV epidemics spike. There were only a few animal model publications from the first isolation of ZIKV from 1947 until 2015, in which only three studies were published as the researchers tested to see the virus pathogenic potential in the animal models (22, 100, 101). That could be a reason why the ZIKV pathogenesis and mechanism are not fully understood yet. In the first publication of a mouse model for ZIKV study, they injected the prototype MR 766 strain of ZIKV into the skull of the mice, which caused neurological disease such as motor weakness and paralysis of the limbs in the suckling or adult mice (22). However, when infecting the adult immunocompetent mice with ZIKV through the intraperitoneal route there was no sign of a disease, which must mean that the infecting route into the skull is essential to initiate any successful infection (99). Recently researchers have looked at ZIKV infection and disease through non-pregnant and pregnant immunocompetent mice as the mice are infected with contemporary ZIKV strains (99). Through ZIKV injection in mice, there were some mice who were notable in showing no clinical disease, and very little or no sign of ZIKV was detected in the wild-type (WT) C57BL/6, Swiss Webster, BALB/c, and CD-1 mice (69, 94, 102, 103). During these outbreaks, there was a disparity in epidemiology and disease presentation as researchers were called onto developed animal models of ZIKV infection and pathogenesis using different strains of ZIKV which similar results are reported in a 2017 review (93). Although ZIKV does not adapt to replicate in immunocompetent mice naturally, as it is missing part of species-specific immune evasion mechanism, the use of immunocompetent mice in ZIKV research is limited. Nevertheless, immunocompetent mice still have an important use for research as immunocompetent mice have been used to evaluate the immunogenicity of vaccines and their protective efficacy from viremia (93, 103).
Immunocompromised mouse models
Although the immunocompetent mouse models cannot support ZIKV replication and disease naturally several groups have looked at the possibility of developing ZIKV infection and disease in immunocompromised adult mice. The innate immune system has played a key role as the innate immune system role is spotting viral infections through the recognition of pathogen associated molecular patterns (PAMPs) by the innate immune system receptors that recognize PAMPs are called host patterns recognition receptors (PRRs) (104). When the PRRs and PAMPS interact, this leads to the start of signaling cascades, which build-up to the production of cytokines, involving type I interferon (IFN) because of the transcription factors NF-κB and interferon regulatory factors (IRFs) (105). Various groups have been able to alter IFN responses in their models, as altering IFN responses is important for antiviral defense (99). The receptors of IFN-α and IFN-β, downstream signaling molecules, and signal transducer and activator of transcription 1 (STAT-1) are vital to protect against viral infection (106, 107). The IFN regulatory factor (IRF), particularly IRF3 and IRF7, is vital in making sure the type I IFN response follows the viral infections (107). Type II IFN or IFN-γ will communicate through a distinct receptor, IFNGR1, as the canonical cytokine of adaptive Th1 immunity and is needed to respond to intracellular pathogens (108). A129 mice (129S2 Ifnar1tm1Agt) and Ifnar1−/− C57BL/6 mice or mice deficient in transcription factors IRF3/5/7−/− lack the receptor for type I IFN, which make them vulnerable to both the African and Asian lineage of ZIKV and suffer from high viral doses in the brain (94, 95, 102, 109, 110). When these animals were injected with ZIKV, they developed symptoms including hind-limb weakness, paralysis, and death. More severe and less severe of these immunocompromised mice after being infected with ZIKV infection depended on their age, as older mice (11-week-old) are less prone to infection than younger mice (3–5-week-old) (94, 102). Mice who did not have both the type I and type II IFN receptors (AG129, 129/Sv Ifnar1tm1Agt Ifngr1tm1Agt) reveal greater severity of diseases after being infected with ZIKV than their counterpart A129 mice (102, 111–113). Through the analysis of the tissues from ZIKV infection A129 and AG129 mice, the most amount of ZIKV doses was in the testes and brain. Having ZIKV present in the testes is consistent with reports of ZIKV mode of transmission as one way would be the sexual transmission. In addition, signal transducer and activator of transcription 2 (Stat2)−/− mice are highly likely to get ZIKV infection. After ZIKV is injected into them, Stat2−/− mice show neurological symptoms as ZIKV was found in the central nervous system (CNS), gonads, and other visceral organs (114). Stat2−/− mice did not have both type 1 and type III IFN signaling. ZIKV infection from an Asian strain in pregnant Ifnar1−/− C57BL/6 gestation lasted from days 6.5 and 7.5 in which some fetal death occurred, and those who survived the ZIKV infection would have problems within their uterus such as growth restriction and growth impairment (68). For these experiments, Ifnar−/−were in the same room with WT sires as they were mated, which resulted in fetuses that were heterozygous for IFNAR1. Although the fetuses can respond to type I IFN, there were still some severe outcomes when observed, implying that having a type 1 IFN response in the fetus still did not protect the fetus from ZIKV induced injury (68).
All in all, these immunocompromised mouse models have been able to show ZIKV ability to cause fetal abnormalities, deterioration of gonadal tissue, and sexual transmission infection (35, 68, 91, 93, 115, 116). Also, these mouse models have been used heavily to continue to understand the pathogenesis and mechanisms to one day create a vaccine for ZIKV (93, 117–121). Keep in mind that although these immunocompromised mouse models are helpful to understand more about ZIKV pathogenesis and mechanism and eventually create a vaccine, there is still a limitation to the models that must be considered when setting up experimental design and looking over the data.
Postnatal infection of ZIKV in mice
Indeed, recent in vivo studies using mouse models and human case studies revealed that ZIKV can pass through the placental barrier to infect the fetus and the infected fetus may die or develop microcephaly or other malformations of the brain (113, 122, 123). Most of the mouse models are used to study congenital infection of ZIKV; it is unknown whether an infection of ZIKV in a newborn baby (a postnatal infection) could cause severe diseases. The results of ours and other groups showed that ZIKV not only replicated in neonatal mice (wild type), but also caused deaths of these mice (6, 71, 94, 95, 124–126). Our recent studies (126) showed that 1) several strains of wild type (wt) neonatal mouse are susceptible to ZIKV infection via different infection routes; and 2) ZIKV infection causes 100% mortalities of 1- to 3-day-old mice, 50–70% mortalities of 5- to 7-day-old mice, and no deaths of 14-day-old or older mice. However, it remains unknown how the ZIKV infection causes deaths of neonatal mice. A neonatal mouse model might be useful to uncover whether and how the ZIKV can infect newborns to cause disease. ZIKV infection in neonatal mice has been reported previously by different groups (6, 71, 94, 95, 124, 125). Our unpublished data show that ZIKV infection in neonatal mice resulted in heart diseases accompanied by abnormal EKG, degradation of gap junction protein alpha (Cx43, aka GJA1). The EKG exhibited 1) P-R extended, implying a block of Atrioventricular conductance; 2) QRS widened, suggesting an intraventricular conductance block; 3) S-T elevation, meaning a myocardial injure. Those are the characteristic of myocardial infarction (127) resulted from necrosis. We hypothesize that ZIKV infection induces the impairing of the function of the neonatal heart and leading to the sudden deaths.
Large animal models of ZIKV infection
Non-human primate (NHP) models
Researchers are also using the non-human primate (NHP) models in studying ZIKV because NHP is more viable in understanding the pathogenesis and mechanism of ZIKV and eventually create a vaccine because of their genetic similarities to humans. Monkeys have a close DNA similarity with humans, so some researchers are studying the rhesus macaques as the rhesus macaques have shown to be susceptible to both the African Strain and Asian Strain of the ZIKV (93, 96, 128–130). When rhesus macaques were infected with ZIKV they developed viremia that rose 2 to 6 days post-infection as when 10 days came about the viremia was undetectable. ZIKV showed up in various parts of the body, such as in the organs, urine, saliva, and cerebrospinal fluid of some animals (96, 128). There was also an interesting finding as several tissues of cynomolgus macaques were found to have been infected with ZIKV which included the male reproductive tract, intestines, brain, and spinal cord (131, 132). ZIKV infected rhesus macaques who were pregnant started to develop viremia, which lasted from 30 to 55 days (96, 128, 129). The subcutaneous injection of an Asian-lineage strain of ZIKV in a pregnant pigtail macaque resulted in her child suffering from a reduced growth of the brain (133, 134). When they analyze the fetal brain, ZIKV infection has caused damage to the central nervous system (CNS). ZIKV was visible in the placenta, fetal brain and liver, and maternal brain, eyes, spleen, and liver (134). In their study, the researchers saw how ZIKV infection prompted a T-cell response to help protect NHPs from being re-infected with ZIKV again and from heterologous ZIKV infection, too (96, 131). Therefore, NHPs are so valuable for being used in testing to develop a vaccine eventually and to test the protective efficacy of the ZIKV vaccine before distributing. In summary, the NHP models are vital in understanding the pathogenesis and mechanism of ZIKV and eventually developing a vaccine as both NHPs and human DNA are very similar. Although the NHP models are great for studying the ZIKV, the expensive NHP models are costly to maintain. They will require ample space to fit each NHPs compared to other animal models such as the small animal models.
ZIKV DIAGNOSTICS
Finding the best diagnostic test for detecting ZIKV is dependent on the stage of the disease as it is divided into two phases: acute and convalescent (135). During the acute phase, there is an infection during the early stages where the viruses will replicate in the infected cells causing the host to develop viremia. When the clinical manifestations are completed, there will be an initial response to the infection as IgM antibodies production builds up to fight against the virus. This immune response is also part of the acute phase. The convalescent phase is the opposite of the acute phase as the convalescent phase begins during the late stage where there is an infection as IgG antibodies are more specific and determined in their response to fight off the developing virus. An ideal diagnostic test should consist of high sensitivity and specificity for the test to correctly assess an individual positive with the disease and correctly assess an individual who is negative for the disease (135). The diagnostic test varies from molecular or serological assays. The molecular assay is mainly used for the detection of genetic variants. Whereas the serological assay can be used to detect the previous circulating virus and measure how much the patient’s immune response against the virus by detecting the antibodies from the opposing virus in the serum (135). Using only clinical evaluation is not sufficient to truly understand ZIKV diagnosis as there is too much cross-reactivity with other arboviruses. Therefore, the Center for Disease Control and Prevention (CDC) has established guidelines for ZIKV diagnosis. The ZIKV diagnostic per CDC guideline consist of a reverse transcriptase reaction assays followed by real-time polymerase chain reaction (RT-PCR), IgM antibody capture enzyme-linked immunosorbent assay (MAC-ELISA), and plaque reduction neutralization test (PRNT).
The RT-PCR assay is a reliable diagnostic method. However, RT-PCR is only suitable when the infection is still in the acute phase because the viral RNA can still be identified in the body fluids (135). RT-PCR assay sensitivity is important in avoiding false negative results. However, limitations exist due to the emerging of variants of ZIKV. There have already been up to ten nucleotide mismatches identified between the oligonucleotide sequences being reported in published assays and the consensus sequence of the Asian lineage ZIKV strain; in addition, there are up to five mismatches in individual primers or probes. Having the inconsistencies of five mismatched primers or probes could be a potential limiting factor for the sensitivity of the RT-PCR as the Asian lineage strain of ZIKV has the genetic variability (136, 137). In summary, there must be continuous research to detect new ZIKV variants and update the primer and probe sequences so that there won’t be any confusion, as the update will help improve the detection sensitivity.
The MAC-ELISA, as a serologic test, is a qualitative detection of antibodies in the serum or cerebrospinal fluid. One downside is that the MAC-ELISA results can be complex to understand due to the possible non-specific reactivity of antibodies. Therefore, the tests that are positive, ambiguous, or inconclusive should be confirmed from the PRNT, a serological test that has the ability to use a specific antibody to neutralize a virus by preventing plaque formation from happening in a cell monolayer. As PRNT is currently considered the gold standard in having the ability to differentiate flavivirus serological diagnosis because of the high specificity. Although this assay is good to use, there is a downside to PRNT because of the high cost involved, such as highly specialized laboratories with proper equipment to take care of cell culture, and the most crucial part is to have permission to work with the active virus. Also training will be needed in learning how to safely use and handle this assay as it is more complex to perform, and the results usually come in 5 to 10 days (137, 138).
There are also some commercial serological tests available, but commercial serological tests from Euroimmun AG (Germany) and InBios (USA) are impressive. The first available serological commercial test was the Euroimmun assay for ZIKV detection, and the assay has been highly evaluated in the literature (139–142). The anti-ZIKV IgM/IgG/IgA ELISA is based on an ELISA using the NS1 protein of ZIKV to detect the IgM, IgG, and IgA in the serum samples. According to Huzly et al (139) studies, there have been reports of high specificity on this test from using various samples from patients with previous exposure to flavivirus infections. In truly understanding which tests work the best there must be a comparative of tests. L’Huillier et al (140) did a comparative study between Euroimmun IgM and IgG ELISAs and MAC-ELISA and subsequent PRNT to confirm whether the results are positive or inconclusive. It is noteworthy that the Euroimmun’s combined IgG/IgM test presented good specificity (95%) as it was better than that of MAC-ELISA. Still the sensitivity of this test was way lower than its counterpart of MAC-ELISA (39.5%).
The InBios assay, also referred to as the ZIKV Detect™ 2.0 IgM Capture ELISA Kit, is an assay for in vitro diagnostic use only. The ZIKV Detect™ 2.0 IgM Capture ELISA Kit is used for qualitative detection of ZIKV IgM antibodies in human sera. This assay was the first commercial serology kit to receive FDA Marketing Authorization in the USA, granted on May 23, 2019. The ZIKV Detect™ 2.0 IgM Capture ELISA Kit recently replaced the ZIKV Detect™ IgM Capture ELISA Kit, and there were numerous significant changes made from the original. According to Basile et al (143) the average sensitivity increased from 90.4% to 92.5% with the ZIKV Detect™ 2.0 IgM Capture ELISA Kit, and the specificity increased from 79.5%to 97.4%, an important 17.9% difference. Also, the accuracy of the updated kit was 89% compared to 63.9% which is a significant 25.1% difference from the original kit, and the agreement among the laboratories increased from 79.5% to 97.4% which is a significant 17.5% difference. In summary, these data have shown that the ZIKV Detect™ 2.0 IgM Capture ELISA Kit can be a successful in vitro diagnostic test and should be looked further into as the ZIKV Detect™ 2.0 IgM Capture ELISA Kit could decrease the amount of PRNT confirmation tests. Overall, all these assays have great potential in being used in routine diagnostic laboratories if they pass each stage in a systematic clinical evaluation.
CONCLUSIONS AND PERSPECTIVES
ZIKV epidemic has put the world on notice, but there has been no clear-cut understanding of ZIKV pathogenesis, prevention, and treatment throughout the years. In the near future, these things will be essential to understand more about ZIKV pathogenesis so that there is a clear-cut plan of prevention and treatment put into place. The mechanisms of how ZIKV infection causes congenital microcephaly and Guillain-Barré syndrome still need to be scrutinized. Animal models will continue to be an essential source for researchers to use in studying ZIKV pathogenesis and progression. When ZIKV pathogenesis is understood, creating a vaccine will be the most logical as with any other viruses. Researchers may have to look into the ZIKV family and genus to build on some of the successful experiences in those viruses as they develop ZIKV vaccine. Once the ZIKV vaccine is developed, the researchers need to test the vaccine on the animal models to make sure it is effective before it is approved to be distributed to the general population.
Some critical questions concerning ZIKV still remain. First, only 5% of the neonates who were born to the ZIKV-infected mothers during pregnancy develop microcephaly. What are the non-viral factors that help ZIKV to cause neurodegeneration? Secondly, although it is demonstrated that Asian strain is more pathogenic than African strain, the two strains exhibited similar effects on cells. A larger number of strains should be applied for infection in cell (2D or 3D) and animals to assess the pathogenic effects of ZIKVs to obtain a more definitive answer. Thirdly, while it is widely accepted that ZIKV infection induced impaired growth and deaths of NPCs, which may be the major pathway to explain the ZIKV-associated microcephaly, ZIKV-caused defective mitosis of cells may play a role as we and others consistently found that ZIKV caused degradation of pericentral materials 1 (PCM1) or structural damage of centrosome. These experimental results still lack a support from animal studies. In addition, we should investigate whether ZIKV infection is related to functional failures of systems other than CNS. For example, can ZIKV infection affect heart development to cause heart diseases? Lastly, ZIKV epidemic has been over for many years. ZIKV infection is now rarely reported. Should it be expected to come back? A long-term prospective investigation for the previously infected patients should be now analyzed to see if the ZIKV has a long effect on humans.
ACKNOWLEDGEMENTS
This study was supported by an NIH/NIAID SC1AI112785 (Q.T.), National Institute on Minority Health/Health Disparities of the National Institutes of Health under Award Number G12MD007597 (Q.T.), and the Howard University Leadership Alliance Summer program (D.V.).
Biography

Qiyi Tang (Ph.D.) is a tenured Professor in the Department of Microbiology at Howard University (HU) College of Medicine. He was trained as a virologist and has been investigating different viruses (Herpesviruses, Flaviviruses and Coronaviruses) for more than 25 years with more than 80 peer-reviewed publications. He has been serving as the Chairperson of Institutional Biosafety Committee at HU (since 2016) and the Cluster co-leader of Infectious and Immune Diseases (IID) in RTRN (RCMI Translational Research Network) in the USA (since 2015). His group and research have been supported by funding from NIH, American Cancer Society (ACS), Charles and Mary Latham Fund and American heart association (AHA). He has been holding memberships in American Society for Microbiology and American Society for Biochemistry and Molecular Biology. He was awarded by Howard University College of medicine as the outstanding faculty researcher of the year (2018) and awarded by ACS as the First researcher in Puerto Rico to receive an ACS Scholar grant, San Juan, PR (2009).
Footnotes
COMPETING INTERESTS
The authors have no other competing interests to disclose.
References:
- 1.Bollati M, Alvarez K, Assenberg R, Baronti C, Canard B, Cook S, Coutard B, Decroly E, de Lamballerie X, Gould EA, Grard G, Grimes JM, Hilgenfeld R, Jansson AM, Malet H, Mancini EJ, Mastrangelo E, Mattevi A, Milani M, Moureau G, Neyts J, Owens RJ, Ren J, Selisko B, Speroni S, Steuber H, Stuart DI, Unge T, Bolognesi M. Structure and functionality in flavivirus NS-proteins: perspectives for drug design. Antiviral Res. 2010;87(2):125–48. doi: 10.1016/j.antiviral.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gatherer D, Kohl A. Zika virus: a previously slow pandemic spreads rapidly through the Americas. J Gen Virol. 2016;97(2):269–73. Epub 2015/12/20. doi: 10.1099/jgv.0.000381. [DOI] [PubMed] [Google Scholar]
- 3.McDougall WM, Perreira JM, Hung HF, Vertii A, Xiaofei E, Zimmerman W, Kowalik TF, Doxsey S, Brass AL. Viral Infection or IFN-alpha Alters Mitotic Spindle Orientation by Modulating Pericentrin Levels. iScience. 2019;12:270–9. Epub 2019/02/05. doi: 10.1016/j.isci.2019.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mlakar J, Korva M, Tul N, Popovic M, Poljsak-Prijatelj M, Mraz J, Kolenc M, Resman Rus K, Vesnaver Vipotnik T, Fabjan Vodusek V, Vizjak A, Pizem J, Petrovec M, Avsic Zupanc T. Zika Virus Associated with Microcephaly. N Engl J Med. 2016;374(10):951–8. doi: 10.1056/NEJMoa1600651. [DOI] [PubMed] [Google Scholar]
- 5.Network. NBDP. Major birth defects data from populationbased birth defects surveillance programs in the United States. Birth Defects Research (Part A): Clinical and Molecular Teratology. 2013;97:S1–S172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang F, Wang HJ, Wang Q, Liu ZY, Yuan L, Huang XY, Li G, Ye Q, Yang H, Shi L, Deng YQ, Qin CF, Xu Z. American Strain of Zika Virus Causes More Severe Microcephaly Than an Old Asian Strain in Neonatal Mice. EBioMedicine. 2017;25:95–105. doi: 10.1016/j.ebiom.2017.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li C, Xu D, Ye Q, Hong S, Jiang Y, Liu X, Zhang N, Shi L, Qin CF, Xu Z. Zika Virus Disrupts Neural Progenitor Development and Leads to Microcephaly in Mice. Cell Stem Cell. 2016;19(5):672. doi: 10.1016/j.stem.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 8.Wang S, Hong S, Deng YQ, Ye Q, Zhao LZ, Zhang FC, Qin CF, Xu Z. Transfer of convalescent serum to pregnant mice prevents Zika virus infection and microcephaly in offspring. Cell Res. 2017;27(1):158–60. doi: 10.1038/cr.2016.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shao Q, Herrlinger S, Yang SL, Lai F, Moore JM, Brindley MA, Chen JF. Zika virus infection disrupts neurovascular development and results in postnatal microcephaly with brain damage. Development. 2016;143(22):4127–36. doi: 10.1242/dev.143768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qian X, Nguyen HN, Song MM, Hadiono C, Ogden SC, Hammack C, Yao B, Hamersky GR, Jacob F, Zhong C, Yoon KJ, Jeang W, Lin L, Li Y, Thakor J, Berg DA, Zhang C, Kang E, Chickering M, Nauen D, Ho CY, Wen Z, Christian KM, Shi PY, Maher BJ, Wu H, Jin P, Tang H, Song H, Ming GL. Brain-Region-Specific Organoids Using Mini-bioreactors for Modeling ZIKV Exposure. Cell. 2016;165:1238–54. doi: 10.1016/j.cell.2016.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcez PP, Loiola EC, Madeiro da Costa R, Higa LM, Trindade P, Delvecchio R, Nascimento JM, Brindeiro R, Tanuri A, Rehen SK. Zika virus impairs growth in human neurospheres and brain organoids. Science. 2016;352(6287):816–8. doi: 10.1126/science.aaf6116. [DOI] [PubMed] [Google Scholar]
- 12.Dang J, Tiwari SK, Lichinchi G, Qin Y, Patil VS, Eroshkin AM, Rana TM. Zika Virus Depletes Neural Progenitors in Human Cerebral Organoids through Activation of the Innate Immune Receptor TLR3. Cell Stem Cell. 2016;19(2):258–65. doi: 10.1016/j.stem.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wen F, Armstrong N, Hou W, Cruz-Cosme R, Obwolo LA, Ishizuka K, Ullah H, Luo MH, Sawa A, Tang Q. Zika virus increases mind bomb 1 levels, causing degradation of pericentriolar material 1 (PCM1) and dispersion of PCM1-containing granules from the centrosome. J Biol Chem. 2019;294(49):18742–55. Epub 2019/11/02. doi: 10.1074/jbc.RA119.010973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johansson MA, Mier-y-Teran-Romero L, Reefhuis J, Gilboa SM, Hills SL. Zika and the Risk of Microcephaly. N Engl J Med. 2016;375(1):1–4. doi: 10.1056/NEJMp1605367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barreto ML, Barral-Netto M, Stabeli R, Almeida-Filho N, Vasconcelos PF, Teixeira M, Buss P, Gadelha PE. Zika virus and microcephaly in Brazil: a scientific agenda. Lancet. 2016. doi: 10.1016/S0140-6736(16)00545-6. [DOI] [PubMed] [Google Scholar]
- 16.Macnamara FN. Zika virus: a report on three cases of human infection during an epidemic of jaundice in Nigeria. Trans R Soc Trop Med Hyg. 1954;48(2):139–45. Epub 1954/03/01. doi: 10.1016/0035-9203(54)90006-1. [DOI] [PubMed] [Google Scholar]
- 17.Chang C, Ortiz K, Ansari A, Gershwin ME. The Zika outbreak of the 21st century. J Autoimmun. 2016;68:1–13. Epub 2016/03/02. doi: 10.1016/j.jaut.2016.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marcondes CB, Ximenes Mde F. Zika virus in Brazil and the danger of infestation by Aedes (Stegomyia) mosquitoes. Rev Soc Bras Med Trop. 2016;49(1):4–10. Epub 2015/12/23. doi: 10.1590/0037-8682-0220-2015. [DOI] [PubMed] [Google Scholar]
- 19.Li MI, Wong PS, Ng LC, Tan CH. Oral susceptibility of Singapore Aedes (Stegomyia) aegypti (Linnaeus) to Zika virus. PLoS Negl Trop Dis. 2012;6(8):e1792. Epub 2012/09/07. doi: 10.1371/journal.pntd.0001792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Plourde AR, Bloch EM. A Literature Review of Zika Virus. Emerg Infect Dis. 2016;22(7):1185–92. doi: 10.3201/eid2207.151990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dick GW, Kitchen SF, Haddow AJ Zika virus I Isolations and serological specificity. Trans R Soc Trop Med Hyg. 1952;46(5):509–20. [DOI] [PubMed] [Google Scholar]
- 22.Dick GW. Zika virus. II. Pathogenicity and physical properties. Trans R Soc Trop Med Hyg. 1952;46(5):521–34. [DOI] [PubMed] [Google Scholar]
- 23.Akoua-Koffi C, Diarrassouba S, Benie VB, Ngbichi JM, Bozoua T, Bosson A, Akran V, Carnevale P, Ehouman A. [Investigation surrounding a fatal case of yellow fever in Cote d’Ivoire in 1999]. Bull Soc Pathol Exot. 2001;94(3):227–30. [PubMed] [Google Scholar]
- 24.Berthet N, Nakoune E, Kamgang B, Selekon B, Descorps-Declere S, Gessain A, Manuguerra JC, Kazanji M. Molecular characterization of three Zika flaviviruses obtained from sylvatic mosquitoes in the Central African Republic. Vector Borne Zoonotic Dis. 2014;14(12):862–5. doi: 10.1089/vbz.2014.1607. [DOI] [PubMed] [Google Scholar]
- 25.Diallo D, Sall AA, Diagne CT, Faye O, Faye O, Ba Y, Hanley KA, Buenemann M, Weaver SC, Diallo M. Zika virus emergence in mosquitoes in southeastern Senegal, 2011. PLoS One. 2014;9(10):e109442. Epub 2014/10/14. doi: 10.1371/journal.pone.0109442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fagbami AH. Zika virus infections in Nigeria: virological and seroepidemiological investigations in Oyo State. J Hyg (Lond). 1979;83(2):213–9. Epub 1979/10/01. doi: 10.1017/s0022172400025997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grard G, Caron M, Mombo IM, Nkoghe D, Mboui Ondo S, Jiolle D, Fontenille D, Paupy C, Leroy EM. Zika virus in Gabon (Central Africa)--2007: a new threat from Aedes albopictus? PLoS Negl Trop Dis. 2014;8(2):e2681. Epub 2014/02/12. doi: 10.1371/journal.pntd.0002681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haddow AJ, Williams MC, Woodall JP, Simpson DI, Goma LK. Twelve Isolations of Zika Virus from Aedes (Stegomyia) Africanus (Theobald) Taken in and above a Uganda Forest. Bull World Health Organ. 1964;31:57–69. Epub 1964/01/01. [PMC free article] [PubMed] [Google Scholar]
- 29.Marchette NJ, Garcia R, Rudnick A. Isolation of Zika virus from Aedes aegypti mosquitoes in Malaysia. Am J Trop Med Hyg. 1969;18(3):411–5. Epub 1969/05/01. doi: 10.4269/ajtmh.1969.18.411. [DOI] [PubMed] [Google Scholar]
- 30.McCrae AW, Kirya BG. Yellow fever and Zika virus epizootics and enzootics in Uganda. Trans R Soc Trop Med Hyg. 1982;76(4):552–62. Epub 1982/01/01. doi: 10.1016/0035-9203(82)90161-4. [DOI] [PubMed] [Google Scholar]
- 31.Souza-Neto JA, Powell JR, Bonizzoni M. Aedes aegypti vector competence studies: A review. Infect Genet Evol. 2019;67:191–209. Epub 2018/11/23. doi: 10.1016/j.meegid.2018.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sakkas H, Bozidis P, Giannakopoulos X, Sofikitis N, Papadopoulou C. An Update on Sexual Transmission of Zika Virus. Pathogens. 2018;7(3). Epub 2018/08/08. doi: 10.3390/pathogens7030066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Foy BD, Kobylinski KC, Chilson Foy JL, Blitvich BJ, Travassos da Rosa A, Haddow AD, Lanciotti RS, Tesh RB. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg Infect Dis. 2011;17(5):880–2. doi: 10.3201/eid1705.101939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moreira J, Peixoto TM, Siqueira AM, Lamas CC. Sexually acquired Zika virus: a systematic review. Clin Microbiol Infect. 2017;23(5):296–305. Epub 2017/01/08. doi: 10.1016/j.cmi.2016.12.027. [DOI] [PubMed] [Google Scholar]
- 35.Tang WW, Young MP, Mamidi A, Regla-Nava JA, Kim K, Shresta S. A Mouse Model of Zika Virus Sexual Transmission and Vaginal Viral Replication. Cell Rep. 2016;17(12):3091–8. Epub 2016/12/24. doi: 10.1016/j.celrep.2016.11.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aquaculture Genomics G, Breeding W, Abdelrahman H, ElHady M, Alcivar-Warren A, Allen S, Al-Tobasei R, Bao L, Beck B, Blackburn H, Bosworth B, Buchanan J, Chappell J, Daniels W, Dong S, Dunham R, Durland E, Elaswad A, Gomez-Chiarri M, Gosh K, Guo X, Hackett P, Hanson T, Hedgecock D, Howard T, Holland L, Jackson M, Jin Y, Khalil K, Kocher T, Leeds T, Li N, Lindsey L, Liu S, Liu Z, Martin K, Novriadi R, Odin R, Palti Y, Peatman E, Proestou D, Qin G, Reading B, Rexroad C, Roberts S, Salem M, Severin A, Shi H, Shoemaker C, Stiles S, Tan S, Tang KF, Thongda W, Tiersch T, Tomasso J, Prabowo WT, Vallejo R, van der Steen H, Vo K, Waldbieser G, Wang H, Wang X, Xiang J, Yang Y, Yant R, Yuan Z, Zeng Q, Zhou T. Aquaculture genomics, genetics and breeding in the United States: current status, challenges, and priorities for future research. BMC Genomics. 2017;18(1):191. Epub 2017/02/22. doi: 10.1186/s12864-017-3557-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Teixeira FME, Pietrobon AJ, Oliveira LM, Oliveira L, Sato MN. Maternal-Fetal Interplay in Zika Virus Infection and Adverse Perinatal Outcomes. Front Immunol. 2020;11:175. Epub 2020/03/03. doi: 10.3389/fimmu.2020.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guedes GR, Coutinho RZ, Marteleto L, Pereira WHS, Duarte D. Why social perception matters during disease outbreaks: looking at how individuals understand the Zika virus by self-reported history of infection. Cad Saude Publica. 2018;34(9):e00139718. Epub 2018/10/04. doi: 10.1590/0102-311X00139718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harris-Sagaribay L, Chambers CD, Perrotta K, Polen KD, Honein MA, Wasternack E. A model partnership for communication and dissemination of scientific recommendations for pregnant women during the emergency response to the Zika virus outbreak: MotherToBaby and the Centers for Disease Control and Prevention. Birth Defects Res. 2020;112(18):1545–50. Epub 2020/08/26. doi: 10.1002/bdr2.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pang W, Lin YL, Xin R, Chen XX, Lu Y, Zheng CB, Yang LM, Zheng YT. Zika virus transmission via breast milk in suckling mice. Clin Microbiol Infect. 2021;27(3):469 e1–e7. Epub 2020/04/29. doi: 10.1016/j.cmi.2020.04.021. [DOI] [PubMed] [Google Scholar]
- 41.In: nd, editor. Guideline: infant feeding in areas of Zika virus transmission. Geneva: 2021. [PubMed] [Google Scholar]
- 42.Guideline: Infant Feeding in Areas of Zika Virus Transmission. Geneva: 2016. [PubMed] [Google Scholar]
- 43.Hayes EB. Zika virus outside Africa. Emerg Infect Dis. 2009;15(9):1347–50. doi: 10.3201/eid1509.090442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Song BH, Yun SI, Woolley M, Lee YM. Zika virus: History, epidemiology, transmission, and clinical presentation. J Neuroimmunol. 2017;308:50–64. Epub 2017/03/14. doi: 10.1016/j.jneuroim.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 45.Haddow AD, Schuh AJ, Yasuda CY, Kasper MR, Heang V, Huy R, Guzman H, Tesh RB, Weaver SC. Genetic characterization of Zika virus strains: geographic expansion of the Asian lineage. PLoS Negl Trop Dis. 2012;6(2):e1477. doi: 10.1371/journal.pntd.0001477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pettersson JH, Bohlin J, Dupont-Rouzeyrol M, Brynildsrud OB, Alfsnes K, Cao-Lormeau VM, Gaunt MW, Falconar AK, de Lamballerie X, Eldholm V, Musso D, Gould EA. Re-visiting the evolution, dispersal and epidemiology of Zika virus in Asia. Emerg Microbes Infect. 2018;7(1):79. Epub 2018/05/10. doi: 10.1038/s41426-018-0082-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Olson JG, Ksiazek TG, Suhandiman, Triwibowo. Zika virus, a cause of fever in Central Java, Indonesia. Trans R Soc Trop Med Hyg. 1981;75(3):389–93. [DOI] [PubMed] [Google Scholar]
- 48.Baronti C, Piorkowski G, Charrel RN, Boubis L, Leparc-Goffart I, de Lamballerie X. Complete coding sequence of zika virus from a French polynesia outbreak in 2013. Genome Announc. 2014;2(3). doi: 10.1128/genomeA.00500-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Faye O, Freire CC, Iamarino A, Faye O, de Oliveira JV, Diallo M, Zanotto PM, Sall AA. Molecular evolution of Zika virus during its emergence in the 20(th) century. PLoS Negl Trop Dis. 2014;8(1):e2636. doi: 10.1371/journal.pntd.0002636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Duffy MR, Chen TH, Hancock WT, Powers AM, Kool JL, Lanciotti RS, Pretrick M, Marfel M, Holzbauer S, Dubray C, Guillaumot L, Griggs A, Bel M, Lambert AJ, Laven J, Kosoy O, Panella A, Biggerstaff BJ, Fischer M, Hayes EB. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360(24):2536–43. doi: 10.1056/NEJMoa0805715. [DOI] [PubMed] [Google Scholar]
- 51.Hussain A, Ali F, Latiwesh OB, Hussain S. A Comprehensive Review of the Manifestations and Pathogenesis of Zika Virus in Neonates and Adults. Cureus. 2018;10(9):e3290. Epub 2018/11/18. doi: 10.7759/cureus.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meertens L, Labeau A, Dejarnac O, Cipriani S, Sinigaglia L, Bonnet-Madin L, Le Charpentier T, Hafirassou ML, Zamborlini A, Cao-Lormeau VM, Coulpier M, Misse D, Jouvenet N, Tabibiazar R, Gressens P, Schwartz O, Amara A. Axl Mediates ZIKA Virus Entry in Human Glial Cells and Modulates Innate Immune Responses. Cell Rep. 2017;18(2):324–33. doi: 10.1016/j.celrep.2016.12.045. [DOI] [PubMed] [Google Scholar]
- 53.Nobrega GM, Samogim AP, Parise PL, Venceslau EM, Guida JPS, Japecanga RR, Amorim MR, Toledo-Teixeira DA, Forato J, Consonni SR, Costa ML, Proenca-Modena JL, Zika-Unicamp N. TAM and TIM receptors mRNA expression in Zika virus infected placentas. Placenta. 2020;101:204–7. Epub 2020/10/05. doi: 10.1016/j.placenta.2020.09.062. [DOI] [PubMed] [Google Scholar]
- 54.Li F, Wang PR, Qu LB, Yi CH, Zhang FC, Tang XP, Zhang LG, Chen L. AXL is not essential for Zika virus infection in the mouse brain. Emerg Microbes Infect. 2017;6(3):e16. doi: 10.1038/emi.2017.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wells MF, Salick MR, Wiskow O, Ho DJ, Worringer KA, Ihry RJ, Kommineni S, Bilican B, Klim JR, Hill EJ, Kane LT, Ye C, Kaykas A, Eggan K. Genetic Ablation of AXL Does Not Protect Human Neural Progenitor Cells and Cerebral Organoids from Zika Virus Infection. Cell Stem Cell. 2016;19(6):703–8. Epub 2016/12/03. doi: 10.1016/j.stem.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 56.Oliveira Melo AS, Malinger G, Ximenes R, Szejnfeld PO, Alves Sampaio S, Bispo de Filippis AM. Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: tip of the iceberg? Ultrasound Obstet Gynecol. 2016;47(1):6–7. Epub 2016/01/06. doi: 10.1002/uog.15831. [DOI] [PubMed] [Google Scholar]
- 57.Ventura CV, Maia M, Bravo-Filho V, Gois AL, Belfort R Jr. Zika virus in Brazil and macular atrophy in a child with microcephaly. Lancet. 2016;387(10015):228. doi: 10.1016/S0140-6736(16)00006-4. [DOI] [PubMed] [Google Scholar]
- 58.Schuler-Faccini L, Sanseverino M, Vianna F, da Silva AA, Larrandaburu M, Marcolongo-Pereira C, Abeche AM. Zika virus: A new human teratogen? Implications for women of reproductive age. Clin Pharmacol Ther. 2016;100(1):28–30. Epub 2016/04/20. doi: 10.1002/cpt.386. [DOI] [PubMed] [Google Scholar]
- 59.Schuler-Faccini L, Ribeiro EM, Feitosa IM, Horovitz DD, Cavalcanti DP, Pessoa A, Doriqui MJ, Neri JI, Neto JM, Wanderley HY, Cernach M, El-Husny AS, Pone MV, Serao CL, Sanseverino MT, Brazilian Medical Genetics Society-Zika Embryopathy Task F. Possible Association Between Zika Virus Infection and Microcephaly - Brazil, 2015. MMWR Morb Mortal Wkly Rep. 2016;65(3):59–62. Epub 2016/01/29. doi: 10.15585/mmwr.mm6503e2. [DOI] [PubMed] [Google Scholar]
- 60.Calvet G, Aguiar RS, Melo AS, Sampaio SA, de Filippis I, Fabri A, Araujo ES, de Sequeira PC, de Mendonca MC, de Oliveira L, Tschoeke DA, Schrago CG, Thompson FL, Brasil P, Dos Santos FB, Nogueira RM, Tanuri A, de Filippis AM. Detection and sequencing of Zika virus from amniotic fluid of fetuses with microcephaly in Brazil: a case study. Lancet Infect Dis. 2016. doi: 10.1016/S1473-3099(16)00095-5. [DOI] [PubMed] [Google Scholar]
- 61.Brasil P, Pereira JP Jr., Moreira ME, Ribeiro Nogueira RM, Damasceno L, Wakimoto M, Rabello RS, Valderramos SG, Halai UA, Salles TS, Zin AA, Horovitz D, Daltro P, Boechat M, Raja Gabaglia C, Carvalho de Sequeira P, Pilotto JH, Medialdea-Carrera R, Cotrim da Cunha D, Abreu de Carvalho LM, Pone M, Machado Siqueira A, Calvet GA, Rodrigues Baiao AE, Neves ES, Nassar de Carvalho PR, Hasue RH, Marschik PB, Einspieler C, Janzen C, Cherry JD, Bispo de Filippis AM, Nielsen-Saines K. Zika Virus Infection in Pregnant Women in Rio de Janeiro. N Engl J Med. 2016;375(24):2321–34. Epub 2016/03/05. doi: 10.1056/NEJMoa1602412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wen Z, Song H, Ming GL. How does Zika virus cause microcephaly? Genes Dev. 2017;31(9):849–61. Epub 2017/06/02. doi: 10.1101/gad.298216.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tang H, Hammack C, Ogden SC, Wen Z, Qian X, Li Y, Yao B, Shin J, Zhang F, Lee EM, Christian KM, Didier RA, Jin P, Song H, Ming GL. Zika Virus Infects Human Cortical Neural Progenitors and Attenuates Their Growth. Cell Stem Cell. 2016;18(5):587–90. doi: 10.1016/j.stem.2016.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cugola FR, Fernandes IR, Russo FB, Freitas BC, Dias JL, Guimaraes KP, Benazzato C, Almeida N, Pignatari GC, Romero S, Polonio CM, Cunha I, Freitas CL, Brandao WN, Rossato C, Andrade DG, Faria Dde P, Garcez AT, Buchpigel CA, Braconi CT, Mendes E, Sall AA, Zanotto PM, Peron JP, Muotri AR, Beltrao-Braga PC. The Brazilian Zika virus strain causes birth defects in experimental models. Nature. 2016;534(7606):267–71. doi: 10.1038/nature18296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ghouzzi VE, Bianchi FT, Molineris I, Mounce BC, Berto GE, Rak M, Lebon S, Aubry L, Tocco C, Gai M, Chiotto AM, Sgro F, Pallavicini G, Simon-Loriere E, Passemard S, Vignuzzi M, Gressens P, Di Cunto F. ZIKA virus elicits P53 activation and genotoxic stress in human neural progenitors similar to mutations involved in severe forms of genetic microcephaly. Cell Death Dis. 2016;7(10):e2440. Epub 2016/10/28. doi: 10.1038/cddis.2016.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Souza BS, Sampaio GL, Pereira CS, Campos GS, Sardi SI, Freitas LA, Figueira CP, Paredes BD, Nonaka CK, Azevedo CM, Rocha VP, Bandeira AC, Mendez-Otero R, Dos Santos RR, Soares MB. Zika virus infection induces mitosis abnormalities and apoptotic cell death of human neural progenitor cells. Sci Rep. 2016;6:39775. Epub 2016/12/23. doi: 10.1038/srep39775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li Y, Muffat J, Omer A, Bosch I, Lancaster MA, Sur M, Gehrke L, Knoblich JA, Jaenisch R. Induction of Expansion and Folding in Human Cerebral Organoids. Cell Stem Cell. 2017;20(3):385–96 e3. Epub 2017/01/04. doi: 10.1016/j.stem.2016.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Miner JJ, Cao B, Govero J, Smith AM, Fernandez E, Cabrera OH, Garber C, Noll M, Klein RS, Noguchi KK, Mysorekar IU, Diamond MS. Zika Virus Infection during Pregnancy in Mice Causes Placental Damage and Fetal Demise. Cell. 2016;165(5):1081–91. doi: 10.1016/j.cell.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rossi SL, Vasilakis N. Modeling Zika Virus Infection in Mice. Cell Stem Cell. 2016;19(1):4–6. Epub 2016/07/09. doi: 10.1016/j.stem.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 70.Rossi SL, Tesh RB, Azar SR, Muruato AE, Hanley KA, Auguste AJ, Langsjoen RM, Paessler S, Vasilakis N, Weaver SC. Characterization of a Novel Murine Model to Study Zika Virus. Am J Trop Med Hyg. 2016. doi: 10.4269/ajtmh.16-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Manangeeswaran M, Ireland DD, Verthelyi D. Zika (PRVABC59) Infection Is Associated with T cell Infiltration and Neurodegeneration in CNS of Immunocompetent Neonatal C57Bl/6 Mice. PLoS Pathog. 2016;12(11):e1006004. doi: 10.1371/journal.ppat.1006004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wolf B, Diop F, Ferraris P, Wichit S, Busso C, Misse D, Gonczy P. Zika virus causes supernumerary foci with centriolar proteins and impaired spindle positioning. Open Biol. 2017;7(1):160231. Epub 2017/01/20. doi: 10.1098/rsob.160231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ferraris P, Cochet M, Hamel R, Gladwyn-Ng I, Alfano C, Diop F, Garcia D, Talignani L, Montero-Menei CN, Nougairede A, Yssel H, Nguyen L, Coulpier M, Misse D. Zika virus differentially infects human neural progenitor cells according to their state of differentiation and dysregulates neurogenesis through the Notch pathway. Emerg Microbes Infect. 2019;8(1):1003–16. Epub 2019/07/10. doi: 10.1080/22221751.2019.1637283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gabriel E, Ramani A, Karow U, Gottardo M, Natarajan K, Gooi LM, Goranci-Buzhala G, Krut O, Peters F, Nikolic M, Kuivanen S, Korhonen E, Smura T, Vapalahti O, Papantonis A, Schmidt-Chanasit J, Riparbelli M, Callaini G, Kronke M, Utermohlen O, Gopalakrishnan J. Recent Zika Virus Isolates Induce Premature Differentiation of Neural Progenitors in Human Brain Organoids. Cell Stem Cell. 2017;20(3):397–406 e5. Epub 2017/01/31. doi: 10.1016/j.stem.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 75.Kesari AS, Heintz VJ, Poudyal S, Miller AS, Kuhn RJ, LaCount DJ. Zika virus NS5 localizes at centrosomes during cell division. Virology. 2020;541:52–62. Epub 2020/02/15. doi: 10.1016/j.virol.2019.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.White MK, Wollebo HS, David Beckham J, Tyler KL, Khalili K. Zika virus: An emergent neuropathological agent. Ann Neurol. 2016;80(4):479–89. Epub 2016/07/28. doi: 10.1002/ana.24748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brasil P, Sequeira PC, Freitas AD, Zogbi HE, Calvet GA, de Souza RV, Siqueira AM, de Mendonca MC, Nogueira RM, de Filippis AM, Solomon T. Guillain-Barre syndrome associated with Zika virus infection. Lancet. 2016;387(10026):1482. doi: 10.1016/S0140-6736(16)30058-7. [DOI] [PubMed] [Google Scholar]
- 78.Oehler E, Watrin L, Larre P, Leparc-Goffart I, Lastere S, Valour F, Baudouin L, Mallet H, Musso D, Ghawche F. Zika virus infection complicated by Guillain-Barre syndrome--case report, French Polynesia, December 2013. Euro Surveill. 2014;19(9). [DOI] [PubMed] [Google Scholar]
- 79.Fontes BM. Zika virus-related hypertensive iridocyclitis. Arq Bras Oftalmol. 2016;79(1):63. Epub 2016/02/04. doi: 10.5935/0004-2749.20160020. [DOI] [PubMed] [Google Scholar]
- 80.Hou W, Armstrong N, Obwolo LA, Thomas M, Pang X, Jones KS, Tang Q. Determination of the Cell Permissiveness Spectrum, Mode of RNA Replication, and RNA-Protein Interaction of Zika Virus. BMC Infect Dis. 2017;17(1):239. doi: 10.1186/s12879-017-2338-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang Y Biology of the Mi-2/NuRD Complex in SLAC (Stemness, Longevity/Ageing, and Cancer). Gene Regul Syst Bio. 2011;5:1–26. doi: 10.4137/GRSB.S6510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hanners NW, Eitson JL, Usui N, Richardson RB, Wexler EM, Konopka G, Schoggins JW. Western Zika Virus in Human Fetal Neural Progenitors Persists Long Term with Partial Cytopathic and Limited Immunogenic Effects. Cell Rep. 2016;15(11):2315–22. Epub 2016/06/09. doi: 10.1016/j.celrep.2016.05.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liang Q, Luo Z, Zeng J, Chen W, Foo SS, Lee SA, Ge J, Wang S, Goldman SA, Zlokovic BV, Zhao Z, Jung JU. Zika Virus NS4A and NS4B Proteins Deregulate Akt-mTOR Signaling in Human Fetal Neural Stem Cells to Inhibit Neurogenesis and Induce Autophagy. Cell Stem Cell. 2016;19(5):663–71. Epub 2016/08/16. doi: 10.1016/j.stem.2016.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Onorati M, Li Z, Liu F, Sousa AM, Nakagawa N, Li M, Dell’Anno MT, Gulden FO, Pochareddy S, Tebbenkamp AT, Han W, Pletikos M, Gao T, Zhu Y, Bichsel C, Varela L, Szigeti-Buck K, Lisgo S, Zhang Y, Testen A, Gao XB, Mlakar J, Popovic M, Flamand M, Strittmatter SM, Kaczmarek LK, Anton ES, Horvath TL, Lindenbach BD, Sestan N. Zika Virus Disrupts Phospho-TBK1 Localization and Mitosis in Human Neuroepithelial Stem Cells and Radial Glia. Cell Rep. 2016;16(10):2576–92. doi: 10.1016/j.celrep.2016.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Retallack H, Di Lullo E, Arias C, Knopp KA, Laurie MT, Sandoval-Espinosa C, Mancia Leon WR, Krencik R, Ullian EM, Spatazza J, Pollen AA, Mandel-Brehm C, Nowakowski TJ, Kriegstein AR, DeRisi JL. Zika virus cell tropism in the developing human brain and inhibition by azithromycin. Proc Natl Acad Sci U S A. 2016;113(50):14408–13. Epub 2016/12/03. doi: 10.1073/pnas.1618029113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bayless NL, Greenberg RS, Swigut T, Wysocka J, Blish CA. Zika Virus Infection Induces Cranial Neural Crest Cells to Produce Cytokines at Levels Detrimental for Neurogenesis. Cell Host Microbe. 2016;20(4):423–8. Epub 2016/10/04. doi: 10.1016/j.chom.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hamel R, Ferraris P, Wichit S, Diop F, Talignani L, Pompon J, Garcia D, Liegeois F, Sall AA, Yssel H, Misse D. African and Asian Zika virus strains differentially induce early antiviral responses in primary human astrocytes. Infect Genet Evol. 2017;49:134–7. Epub 2017/01/18. doi: 10.1016/j.meegid.2017.01.015. [DOI] [PubMed] [Google Scholar]
- 88.Aagaard KM, Lahon A, Suter MA, Arya RP, Seferovic MD, Vogt MB, Hu M, Stossi F, Mancini MA, Harris RA, Kahr M, Eppes C, Rac M, Belfort MA, Park CS, Lacorazza D, Rico-Hesse R. Primary Human Placental Trophoblasts are Permissive for Zika Virus (ZIKV) Replication. Sci Rep. 2017;7:41389. Epub 2017/01/28. doi: 10.1038/srep41389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Quicke KM, Bowen JR, Johnson EL, McDonald CE, Ma H, O’Neal JT, Rajakumar A, Wrammert J, Rimawi BH, Pulendran B, Schinazi RF, Chakraborty R, Suthar MS. Zika Virus Infects Human Placental Macrophages. Cell Host Microbe. 2016;20(1):83–90. Epub 2016/06/02. doi: 10.1016/j.chom.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bayer A, Lennemann NJ, Ouyang Y, Bramley JC, Morosky S, Marques ET Jr., Cherry S, Sadovsky Y, Coyne CB. Type III Interferons Produced by Human Placental Trophoblasts Confer Protection against Zika Virus Infection. Cell Host Microbe. 2016;19(5):705–12. Epub 2016/04/14. doi: 10.1016/j.chom.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ma W, Li S, Ma S, Jia L, Zhang F, Zhang Y, Zhang J, Wong G, Zhang S, Lu X, Liu M, Yan J, Li W, Qin C, Han D, Qin C, Wang N, Li X, Gao GF. Zika Virus Causes Testis Damage and Leads to Male Infertility in Mice. Cell. 2016;167(6):1511–24 e10. doi: 10.1016/j.cell.2016.11.016. [DOI] [PubMed] [Google Scholar]
- 92.Jagger BW, Miner JJ, Cao B, Arora N, Smith AM, Kovacs A, Mysorekar IU, Coyne CB, Diamond MS. Gestational Stage and IFN-lambda Signaling Regulate ZIKV Infection In Utero. Cell Host Microbe. 2017;22(3):366–76 e3. doi: 10.1016/j.chom.2017.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Morrison TE, Diamond MS. Animal Models of Zika Virus Infection, Pathogenesis, and Immunity. J Virol. 2017;91(8):9–17. doi: 10.1128/JVI.00009-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lazear HM, Govero J, Smith AM, Platt DJ, Fernandez E, Miner JJ, Diamond MS. A Mouse Model of Zika Virus Pathogenesis. Cell Host Microbe. 2016;19(5):720–30. doi: 10.1016/j.chom.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Miner JJ, Sene A, Richner JM, Smith AM, Santeford A, Ban N, Weger-Lucarelli J, Manzella F, Ruckert C, Govero J, Noguchi KK, Ebel GD, Diamond MS, Apte RS. Zika Virus Infection in Mice Causes Panuveitis with Shedding of Virus in Tears. Cell Rep. 2016;16(12):3208–18. doi: 10.1016/j.celrep.2016.08.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dudley DM, Aliota MT, Mohr EL, Weiler AM, Lehrer-Brey G, Weisgrau KL, Mohns MS, Breitbach ME, Rasheed MN, Newman CM, Gellerup DD, Moncla LH, Post J, Schultz-Darken N, Schotzko ML, Hayes JM, Eudailey JA, Moody MA, Permar SR, O’Connor SL, Rakasz EG, Simmons HA, Capuano S, Golos TG, Osorio JE, Friedrich TC, O’Connor DH. A rhesus macaque model of Asian-lineage Zika virus infection. Nat Commun. 2016;7:12204. Epub 2016/06/29. doi: 10.1038/ncomms12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Abbink P, Larocca RA, De La Barrera RA, Bricault CA, Moseley ET, Boyd M, Kirilova M, Li Z, Ng’ang’a D, Nanayakkara O, Nityanandam R, Mercado NB, Borducchi EN, Agarwal A, Brinkman AL, Cabral C, Chandrashekar A, Giglio PB, Jetton D, Jimenez J, Lee BC, Mojta S, Molloy K, Shetty M, Neubauer GH, Stephenson KE, Peron JP, Zanotto PM, Misamore J, Finneyfrock B, Lewis MG, Alter G, Modjarrad K, Jarman RG, Eckels KH, Michael NL, Thomas SJ, Barouch DH. Protective efficacy of multiple vaccine platforms against Zika virus challenge in rhesus monkeys. Science. 2016;353(6304):1129–32. Epub 2016/08/06. doi: 10.1126/science.aah6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hirsch AJ, Smith JL, Haese NN, Broeckel RM, Parkins CJ, Kreklywich C, DeFilippis VR, Denton M, Smith PP, Messer WB, Colgin LM, Ducore RM, Grigsby PL, Hennebold JD, Swanson T, Legasse AW, Axthelm MK, MacAllister R, Wiley CA, Nelson JA, Streblow DN. Zika Virus infection of rhesus macaques leads to viral persistence in multiple tissues. PLoS Pathog. 2017;13(3):e1006219. Epub 2017/03/10. doi: 10.1371/journal.ppat.1006219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Krause KK, Azouz F, Shin OS, Kumar M. Understanding the Pathogenesis of Zika Virus Infection Using Animal Models. Immune Netw. 2017;17(5):287–97. Epub 2017/11/03. doi: 10.4110/in.2017.17.5.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bell TM, Field EJ, Narang HK. Zika virus infection of the central nervous system of mice. Arch Gesamte Virusforsch. 1971;35(2):183–93. Epub 1971/01/01. doi: 10.1007/BF01249709. [DOI] [PubMed] [Google Scholar]
- 101.Way JH, Bowen ET, Platt GS. Comparative studies of some African arboviruses in cell culture and in mice. J Gen Virol. 1976;30(1):123–30. Epub 1976/01/01. doi: 10.1099/0022-1317-30-1-123. [DOI] [PubMed] [Google Scholar]
- 102.Rossi SL, Tesh RB, Azar SR, Muruato AE, Hanley KA, Auguste AJ, Langsjoen RM, Paessler S, Vasilakis N, Weaver SC. Characterization of a Novel Murine Model to Study Zika Virus. Am J Trop Med Hyg. 2016;94(6):1362–9. Epub 2016/03/30. doi: 10.4269/ajtmh.16-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Larocca RA, Abbink P, Peron JP, Zanotto PM, Iampietro MJ, Badamchi-Zadeh A, Boyd M, Ng’ang’a D, Kirilova M, Nityanandam R, Mercado NB, Li Z, Moseley ET, Bricault CA, Borducchi EN, Giglio PB, Jetton D, Neubauer G, Nkolola JP, Maxfield LF, De La Barrera RA, Jarman RG, Eckels KH, Michael NL, Thomas SJ, Barouch DH. Vaccine protection against Zika virus from Brazil. Nature. 2016;536(7617):474–8. Epub 2016/06/30. doi: 10.1038/nature18952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Medzhitov R Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1(2):135–45. Epub 2002/03/22. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 105.Honda K, Taniguchi T. IRFs: master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nat Rev Immunol. 2006;6(9):644–58. Epub 2006/08/26. doi: 10.1038/nri1900. [DOI] [PubMed] [Google Scholar]
- 106.Haller O, Kochs G, Weber F. The interferon response circuit: induction and suppression by pathogenic viruses. Virology. 2006;344(1):119–30. Epub 2005/12/21. doi: 10.1016/j.virol.2005.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Suthar MS, Diamond MS, Gale M Jr., West Nile virus infection and immunity. Nat Rev Microbiol. 2013;11(2):115–28. Epub 2013/01/17. doi: 10.1038/nrmicro2950. [DOI] [PubMed] [Google Scholar]
- 108.Schoenborn JR, Wilson CB. Regulation of interferon-gamma during innate and adaptive immune responses. Adv Immunol. 2007;96:41–101. Epub 2007/11/06. doi: 10.1016/S0065-2776(07)96002-2. [DOI] [PubMed] [Google Scholar]
- 109.Dowall SD, Graham VA, Rayner E, Atkinson B, Hall G, Watson RJ, Bosworth A, Bonney LC, Kitchen S, Hewson R. A Susceptible Mouse Model for Zika Virus Infection. PLoS Negl Trop Dis. 2016;10(5):e0004658. doi: 10.1371/journal.pntd.0004658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li H, Saucedo-Cuevas L, Regla-Nava JA, Chai G, Sheets N, Tang W, Terskikh AV, Shresta S, Gleeson JG. Zika Virus Infects Neural Progenitors in the Adult Mouse Brain and Alters Proliferation. Cell Stem Cell. 2016;19(5):593–8. doi: 10.1016/j.stem.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zmurko J, Marques RE, Schols D, Verbeken E, Kaptein SJ, Neyts J. The Viral Polymerase Inhibitor 7-Deaza-2’-C-Methyladenosine Is a Potent Inhibitor of In Vitro Zika Virus Replication and Delays Disease Progression in a Robust Mouse Infection Model. PLoS Negl Trop Dis. 2016;10(5):e0004695. doi: 10.1371/journal.pntd.0004695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Julander JG, Siddharthan V, Evans J, Taylor R, Tolbert K, Apuli C, Stewart J, Collins P, Gebre M, Neilson S, Van Wettere A, Lee YM, Sheridan WP, Morrey JD, Babu YS. Efficacy of the broad-spectrum antiviral compound BCX4430 against Zika virus in cell culture and in a mouse model. Antiviral Res. 2017;137:14–22. doi: 10.1016/j.antiviral.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Aliota MT, Caine EA, Walker EC, Larkin KE, Camacho E, Osorio JE. Characterization of Lethal Zika Virus Infection in AG129 Mice. PLoS Negl Trop Dis. 2016;10(4):e0004682. doi: 10.1371/journal.pntd.0004682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tripathi S, Balasubramaniam VR, Brown JA, Mena I, Grant A, Bardina SV, Maringer K, Schwarz MC, Maestre AM, Sourisseau M, Albrecht RA, Krammer F, Evans MJ, Fernandez-Sesma A, Lim JK, Garcia-Sastre A. A novel Zika virus mouse model reveals strain specific differences in virus pathogenesis and host inflammatory immune responses. PLoS Pathog. 2017;13(3):e1006258. doi: 10.1371/journal.ppat.1006258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Govero J, Esakky P, Scheaffer SM, Fernandez E, Drury A, Platt DJ, Gorman MJ, Richner JM, Caine EA, Salazar V, Moley KH, Diamond MS. Zika virus infection damages the testes in mice. Nature. 2016;540(7633):438–42. Epub 2016/11/01. doi: 10.1038/nature20556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yockey LJ, Varela L, Rakib T, Khoury-Hanold W, Fink SL, Stutz B, Szigeti-Buck K, Van den Pol A, Lindenbach BD, Horvath TL, Iwasaki A. Vaginal Exposure to Zika Virus during Pregnancy Leads to Fetal Brain Infection. Cell. 2016;166(5):1247–56 e4. doi: 10.1016/j.cell.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bullard-Feibelman KM, Govero J, Zhu Z, Salazar V, Veselinovic M, Diamond MS, Geiss BJ. The FDA-approved drug sofosbuvir inhibits Zika virus infection. Antiviral Res. 2017;137:134–40. Epub 2016/12/03. doi: 10.1016/j.antiviral.2016.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Deng YQ, Zhang NN, Li CF, Tian M, Hao JN, Xie XP, Shi PY, Qin CF. Adenosine Analog NITD008 Is a Potent Inhibitor of Zika Virus. Open Forum Infect Dis. 2016;3(4):ofw175. Epub 2016/10/18. doi: 10.1093/ofid/ofw175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhao H, Fernandez E, Dowd KA, Speer SD, Platt DJ, Gorman MJ, Govero J, Nelson CA, Pierson TC, Diamond MS, Fremont DH. Structural Basis of Zika Virus-Specific Antibody Protection. Cell. 2016;166(4):1016–27. Epub 2016/08/01. doi: 10.1016/j.cell.2016.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Swanstrom JA, Plante JA, Plante KS, Young EF, McGowan E, Gallichotte EN, Widman DG, Heise MT, de Silva AM, Baric RS. Dengue Virus Envelope Dimer Epitope Monoclonal Antibodies Isolated from Dengue Patients Are Protective against Zika Virus. mBio. 2016;7(4). Epub 2016/07/21. doi: 10.1128/mBio.01123-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sapparapu G, Fernandez E, Kose N, Bin C, Fox JM, Bombardi RG, Zhao H, Nelson CA, Bryan AL, Barnes T, Davidson E, Mysorekar IU, Fremont DH, Doranz BJ, Diamond MS, Crowe JE. Neutralizing human antibodies prevent Zika virus replication and fetal disease in mice. Nature. 2016;540(7633):443–7. Epub 2016/11/08. doi: 10.1038/nature20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hickman HD, Pierson TC. Zika in the Brain: New Models Shed Light on Viral Infection. Trends Mol Med. 2016. doi: 10.1016/j.molmed.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lazear HM, Govero J, Smith AM, Platt DJ, Fernandez E, Miner JJ, Diamond MS. A Mouse Model of Zika Virus Pathogenesis. Cell Host Microbe. 2016. doi: 10.1016/j.chom.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fernandes NC, Nogueira JS, Ressio RA, Cirqueira CS, Kimura LM, Fernandes KR, Cunha MS, Souza RP, Guerra JM. Experimental Zika virus infection induces spinal cord injury and encephalitis in newborn Swiss mice. Exp Toxicol Pathol. 2017;69(2):63–71. doi: 10.1016/j.etp.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 125.van den Pol AN, Mao G, Yang Y, Ornaghi S, Davis JN. Zika Virus Targeting in the Developing Brain. J Neurosci. 2017;37(8):2161–75. doi: 10.1523/JNEUROSCI.3124-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li S, Armstrong N, Zhao H, Hou W, Liu J, Chen C, Wan J, Wang W, Zhong C, Liu C, Zhu H, Xia N, Cheng T, Tang Q. Zika Virus Fatally Infects Wild Type Neonatal Mice and Replicates in Central Nervous System. Viruses. 2018;10(1). doi: 10.3390/v10010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mythili S, Malathi N. Diagnostic markers of acute myocardial infarction. Biomed Rep. 2015;3(6):743–8. doi: 10.3892/br.2015.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Li XF, Dong HL, Huang XY, Qiu YF, Wang HJ, Deng YQ, Zhang NN, Ye Q, Zhao H, Liu ZY, Fan H, An XP, Sun SH, Gao B, Fa YZ, Tong YG, Zhang FC, Gao GF, Cao WC, Shi PY, Qin CF. Characterization of a 2016 Clinical Isolate of Zika Virus in Non-human Primates. EBioMedicine. 2016;12:170–7. doi: 10.1016/j.ebiom.2016.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nguyen SM, Antony KM, Dudley DM, Kohn S, Simmons HA, Wolfe B, Salamat MS, Teixeira LBC, Wiepz GJ, Thoong TH, Aliota MT, Weiler AM, Barry GL, Weisgrau KL, Vosler LJ, Mohns MS, Breitbach ME, Stewart LM, Rasheed MN, Newman CM, Graham ME, Wieben OE, Turski PA, Johnson KM, Post J, Hayes JM, Schultz-Darken N, Schotzko ML, Eudailey JA, Permar SR, Rakasz EG, Mohr EL, Capuano S 3rd, Tarantal AF, Osorio JE, O’Connor SL, Friedrich TC, O’Connor DH, Golos TG. Highly efficient maternal-fetal Zika virus transmission in pregnant rhesus macaques. PLoS Pathog. 2017;13(5):e1006378. Epub 2017/05/26. doi: 10.1371/journal.ppat.1006378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Aliota MT, Dudley DM, Newman CM, Mohr EL, Gellerup DD, Breitbach ME, Buechler CR, Rasheed MN, Mohns MS, Weiler AM, Barry GL, Weisgrau KL, Eudailey JA, Rakasz EG, Vosler LJ, Post J, Capuano S 3rd, Golos TG, Permar SR, Osorio JE, Friedrich TC, O’Connor SL, O’Connor DH. Heterologous Protection against Asian Zika Virus Challenge in Rhesus Macaques. PLoS Negl Trop Dis. 2016;10(12):e0005168. Epub 2016/12/03. doi: 10.1371/journal.pntd.0005168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Osuna CE, Lim SY, Deleage C, Griffin BD, Stein D, Schroeder LT, Omange RW, Best K, Luo M, Hraber PT, Andersen-Elyard H, Ojeda EF, Huang S, Vanlandingham DL, Higgs S, Perelson AS, Estes JD, Safronetz D, Lewis MG, Whitney JB. Zika viral dynamics and shedding in rhesus and cynomolgus macaques. Nat Med. 2016;22(12):1448–55. Epub 2016/11/01. doi: 10.1038/nm.4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Koide F, Goebel S, Snyder B, Walters KB, Gast A, Hagelin K, Kalkeri R, Rayner J. Development of a Zika Virus Infection Model in Cynomolgus Macaques. Front Microbiol. 2016;7:2028. Epub 2017/01/10. doi: 10.3389/fmicb.2016.02028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Driggers RW, Ho CY, Korhonen EM, Kuivanen S, Jaaskelainen AJ, Smura T, Rosenberg A, Hill DA, DeBiasi RL, Vezina G, Timofeev J, Rodriguez FJ, Levanov L, Razak J, Iyengar P, Hennenfent A, Kennedy R, Lanciotti R, du Plessis A, Vapalahti O. Zika Virus Infection with Prolonged Maternal Viremia and Fetal Brain Abnormalities. N Engl J Med. 2016;374(22):2142–51. doi: 10.1056/NEJMoa1601824. [DOI] [PubMed] [Google Scholar]
- 134.Adams Waldorf KM, Stencel-Baerenwald JE, Kapur RP, Studholme C, Boldenow E, Vornhagen J, Baldessari A, Dighe MK, Thiel J, Merillat S, Armistead B, Tisoncik-Go J, Green RR, Davis MA, Dewey EC, Fairgrieve MR, Gatenby JC, Richards T, Garden GA, Diamond MS, Juul SE, Grant RF, Kuller L, Shaw DW, Ogle J, Gough GM, Lee W, English C, Hevner RF, Dobyns WB, Gale M Jr., Rajagopal L. Fetal brain lesions after subcutaneous inoculation of Zika virus in a pregnant nonhuman primate. Nat Med. 2016;22(11):1256–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Silva IBB, da Silva AS, Cunha MS, Cabral AD, de Oliveira KCA, Gaspari E, Prudencio CR. Zika virus serological diagnosis: commercial tests and monoclonal antibodies as tools. J Venom Anim Toxins Incl Trop Dis. 2020;26:e20200019. Epub 2020/12/08. doi: 10.1590/1678-9199-JVATITD-2020-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Corman VM, Rasche A, Baronti C, Aldabbagh S, Cadar D, Reusken CB, Pas SD, Goorhuis A, Schinkel J, Molenkamp R, Kummerer BM, Bleicker T, Brunink S, Eschbach-Bludau M, Eis-Hubinger AM, Koopmans MP, Schmidt-Chanasit J, Grobusch MP, de Lamballerie X, Drosten C, Drexler JF. Assay optimization for molecular detection of Zika virus. Bull World Health Organ. 2016;94(12):880–92. Epub 2016/12/21. doi: 10.2471/BLT.16.175950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shi W, Zhang Z, Ling C, Carr MJ, Tong Y, Gao GF. Increasing genetic diversity of Zika virus in the Latin American outbreak. Emerg Microbes Infect. 2016;5:e68. Epub 2016/07/07. doi: 10.1038/emi.2016.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Roehrig JT, Hombach J, Barrett AD. Guidelines for Plaque-Reduction Neutralization Testing of Human Antibodies to Dengue Viruses. Viral Immunol. 2008;21(2):123–32. Epub 2008/05/15. doi: 10.1089/vim.2008.0007. [DOI] [PubMed] [Google Scholar]
- 139.Huzly D, Hanselmann I, Schmidt-Chanasit J, Panning M. High specificity of a novel Zika virus ELISA in European patients after exposure to different flaviviruses. Euro Surveill. 2016;21(16). Epub 2016/04/30. doi: 10.2807/1560-7917.ES.2016.21.16.30203. [DOI] [PubMed] [Google Scholar]
- 140.L’Huillier AG, Hamid-Allie A, Kristjanson E, Papageorgiou L, Hung S, Wong CF, Stein DR, Olsha R, Goneau LW, Dimitrova K, Drebot M, Safronetz D, Gubbay JB. Evaluation of Euroimmun Anti-Zika Virus IgM and IgG Enzyme-Linked Immunosorbent Assays for Zika Virus Serologic Testing. J Clin Microbiol. 2017;55(8):2462–71. Epub 2017/06/02. doi: 10.1128/JCM.00442-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kadkhoda K, Gretchen A, Racano A. Evaluation of a commercially available Zika virus IgM ELISA: specificity in focus. Diagn Microbiol Infect Dis. 2017;88(3):233–5. Epub 2017/05/10. doi: 10.1016/j.diagmicrobio.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 142.Steinhagen K, Probst C, Radzimski C, Schmidt-Chanasit J, Emmerich P, van Esbroeck M, Schinkel J, Grobusch MP, Goorhuis A, Warnecke JM, Lattwein E, Komorowski L, Deerberg A, Saschenbrecker S, Stocker W, Schlumberger W. Serodiagnosis of Zika virus (ZIKV) infections by a novel NS1-based ELISA devoid of cross-reactivity with dengue virus antibodies: a multicohort study of assay performance, 2015 to 2016. Euro Surveill. 2016;21(50). Epub 2016/12/23. doi: 10.2807/1560-7917.ES.2016.21.50.30426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Basile AJ, Ao J, Horiuchi K, Semenova V, Steward-Clark E, Schiffer J. Performance of InBios ZIKV Detect 2.0 IgM Capture ELISA in two reference laboratories compared to the original ZIKV Detect IgM Capture ELISA. J Virol Methods. 2019;271:113671. Epub 2019/06/11. doi: 10.1016/j.jviromet.2019.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]


