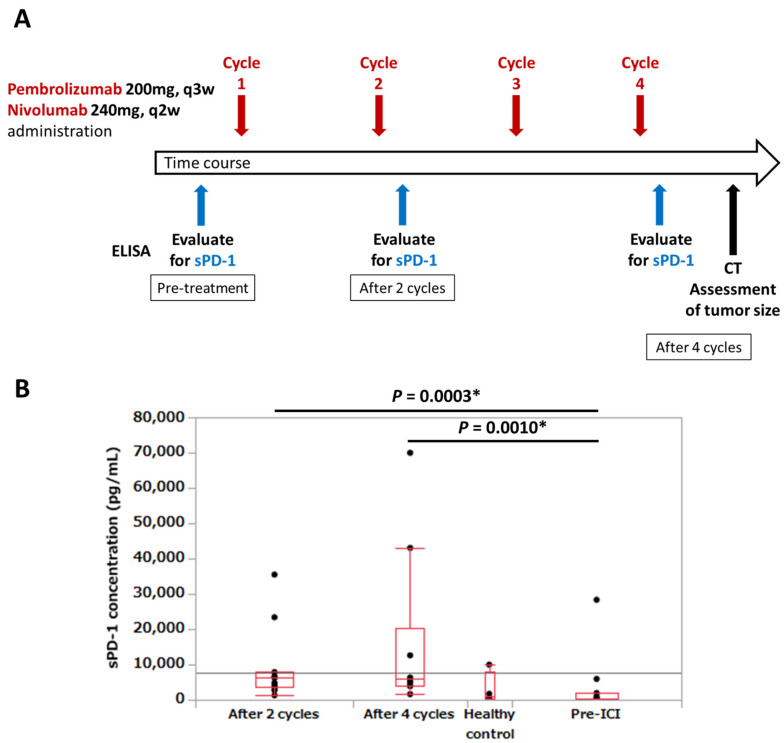
Figure 1.
Plasma sPD-1 concentrations at each treatment point. (A) Schema for schedule of ICI administration and timing of sPD-1 measurement. We retrospectively analyzed data from 22 patients (12 with first-line or previously treated NSCLC, 9 with gastric cancer, and 1 with bladder cancer) who received anti-PD-1 antibody therapy (nivolumab (240 mg) intravenously every 2 weeks or pembrolizumab (200 mg) intravenously every 3 weeks). Plasma levels of sPD-1 were evaluated pretreatment and after two and four cycles of anti-PD-1 antibody therapy. A change in tumor size was defined as the percentage change in tumor size from baseline to after four cycles of anti-PD-1 antibody therapy. (B) Blood samples were collected before and after treatment, and the plasma levels of sPD-1 were measured by en-zyme-linked immunosorbent assay (ELISA). We measured sPD-1 concentration for healthy control subjects (N = 4), and patients administered anti-PD-1 antibodies. For patients, sPD-1 was measured before treatment (N = 15), after 2 cycles (N = 14), and at the point after 4 cycles (N = 10) of ICI administration. We plotted the sPD-1 concentration at each time point and compared each. The levels of sPD-1 were significantly increased after 2 and 4 cycles, compared to pretreat-ment levels (p = 0.0003; p = 0.0010, respectively). * Statistically significant.