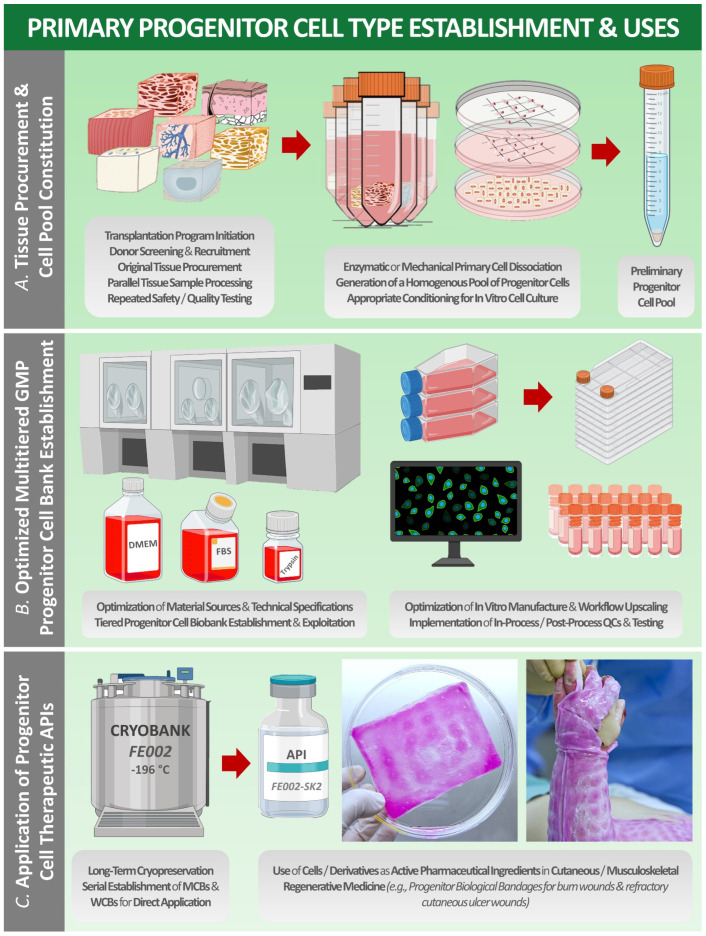
Figure 1.
Schematic technical overview of the multiple steps performed in modern settings for the appropriate sourcing, manufacturing, and formulation of clinically compatible progenitor cell-based therapeutic products or standardized transplants. (A) Tissue procurement and preliminary progenitor cell pool constitution step. (B) Multiparametric technical optimization phase and multitiered GMP biobanking of primary progenitor cells. (C) Example of a clinical application of extemporaneously reconstituted skin-derived progenitor cells (e.g., FE002-SK2 fibroblasts), topically applied as viable cells seeded on a collagen scaffold (e.g., PBB product) for pediatric burn patient care in Lausanne under the Swiss progenitor cell transplantation program. API, active pharmaceutical ingredient; DMEM, Dulbecco’s modified Eagle medium; FBS, fetal bovine serum; GMP, good manufacturing practice; MCB, master cell bank; PBB, progenitor biological bandage; QC, quality control; WCB, working cell bank.