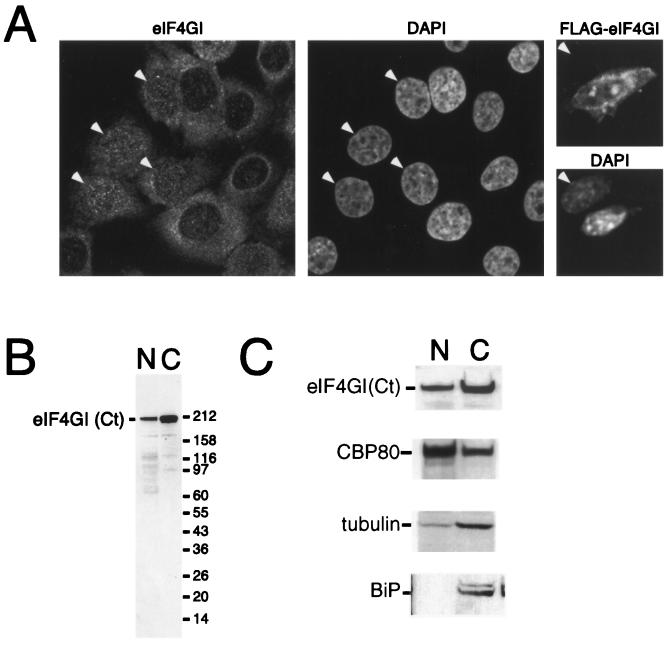
FIG. 3.
eIF4GI is distributed between the nucleus and cytoplasm. (A) HeLa cells were cultured on coverslips and fixed, endogenous eIF4G was localized by using affinity-purified anti-eIF4GI(Ct) antiserum (left), and DNA was stained with DAPI (4′,6′-diamidino-2-phenylindole) (middle). Note that eIF4GI localizes to both the nucleus and the cytoplasm and that some cells display strong nuclear staining (arrowheads). NIH 3T3 cells were grown on coverslips (right) and transfected with a vector encoding FLAG-eIF4G. Expressed eIF4G was localized using an anti-FLAG antibody (upper), and DNA was visualized by DAPI staining (lower). A transfected and a nontransfected (arrowhead) cell are shown side by side; epitope-tagged eIF4GI can be detected in both the nucleus and the cytoplasm of the transfected cell. (B) HeLa cells were separated into nuclear (N) and cytoplasmic (C) fractions, as described in Materials and Methods. The recovery of eIF4G in the nuclear and cytoplasmic fractions was visualized by SDS-PAGE and immunoblotting using an affinity-purified antibody. (C) HeLa cells were separated into nuclear and cytoplasmic fractions as described above. Cell equivalents of the nuclear and cytoplasmic fractions were loaded, separated by SDS-PAGE, and blotted. The recovery of eIF4G [using antisera specific to eIF4G(Ct)], CBP80, BiP, and γ-tubulin in the nuclear and cytoplasmic fractions was monitored by SDS-PAGE and Western blotting using ECL.