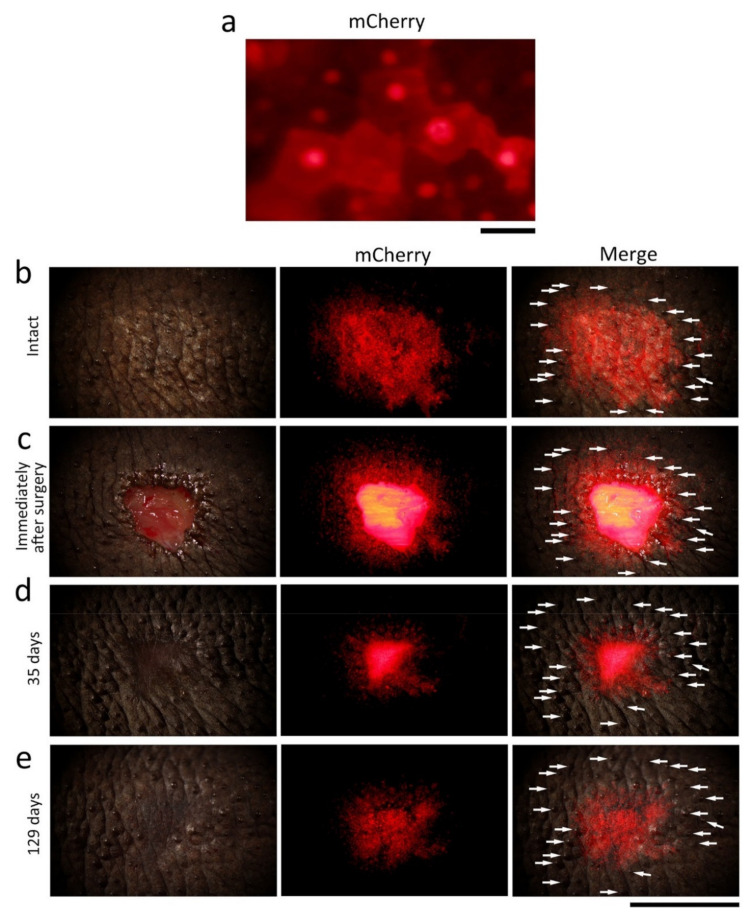
Figure 10.
Tracking of the epidermis around the wound margin during skin regeneration. A mosaic pattern of a transgenic adult newt (CAGGs > mCherry (I-SceI)) was used in which a red fluorescent protein, mCherry, was expressed in spots on the back skin. (a) A magnified view of the stratum corneum in the fluorescent spot under a dissecting microscope. The mCherry fluorescence of cobble stone-shaped epithelial cells made them visible. (b–e) Tracking of the fluorescent epidermis. A central part of the fluorescent spot was excised and then the fluorescent epidermis around the wound was monitored. (b) Before surgery (intact). (c) Immediately after surgery. The wound bed fluoresced because muscles intensely expressed mCherry in this animal. (d) 35 days after surgery. (e) 129 days after surgery. Left-hand column: bright light image. Central column: mCherry fluorescence. Right-hand column: Merge. Arrows in the right-hand column indicate secretion glands as landmarks. We waited for the stratum corneum and the upper part of the transitional layer of the fluorescent epidermis to be renewed (the stratum corneum was shed every two to three weeks) allowing the fluorescent area of the basal layer and the lower part of the transitional layer to be tracked, both of which should slide toward the wound bed according to our hypothesis. Consistently, at 35 days after surgery, the fluorescent area had shrunk compared to the landmarks. The fluorescence of the wound epidermis became recognizable as the wound bed was covered by the pigment cell layer, which blocked off fluorescence from the muscles (129 days). At 129 days, the fluorescent area seemed to have expanded slightly, probably because the skin had relaxed. Note that this experiment was conducted on a single newt, so the same experiment needs to be repeated on multiple individuals to corroborate the results. Scale bars: 40 μm (a); 5 mm (b–e).