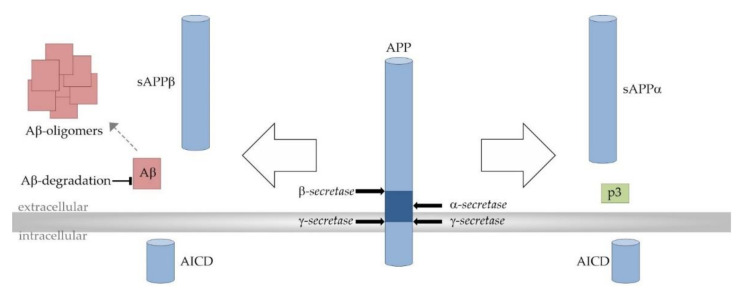
Figure 3.
Proteolytic processing of the amyloid precursor protein (APP). Amyloidogenic APP processing pathway (left): Amyloidogenic APP processing is initiated by β-secretase (β-site APP cleaving enzyme 1, BACE1)-dependent cleavage of APP within its extracellular domain, generating the soluble β-secreted APP (sAPPβ) and the C-terminal membrane-bound fragment C99/β-CTF (APP–β-carboxyl terminal fragment) as intermediate product (not shown). C99/β-CTF is further cleaved by the γ-secretase complex resulting in the release of amyloid-β (Aβ) peptides (red) into the extracellular space. The aggregation of Aβ involves distinct intermediates and finally leads to the generation of larger Aβ aggregates and amyloid plaques (red). Total cerebral Aβ levels and amyloid pathology are also determined by Aβ elimination involving transport processes and its enzymatic degradation. Non-amyloidogenic APP processing pathway (right): In the non-amyloidogenic pathway APP is first processed by the α-secretases (A Disintegrin and metalloproteinase domain-containing proteins 10 and 17, ADAM10 and ADAM17). α-secretase-dependent APP cleavage generates soluble α-secreted APP (sAPPα) and the membrane spanning fragment C83/α-CTF (APP–α-carboxyl terminal fragment) as intermediate (not shown). C83/α-CTF is further cleaved by the γ-secretase complex to generate the non-toxic peptide p3 (green). Since the α-secretase cleavage site in APP is located within the Aβ domain (dark blue), the formation of Aβ peptides is precluded in the non-amyloidogenic APP processing pathway. In both APP processing pathways, the transcriptionally active APP intracellular domain (AICD) is released into the cytosol.