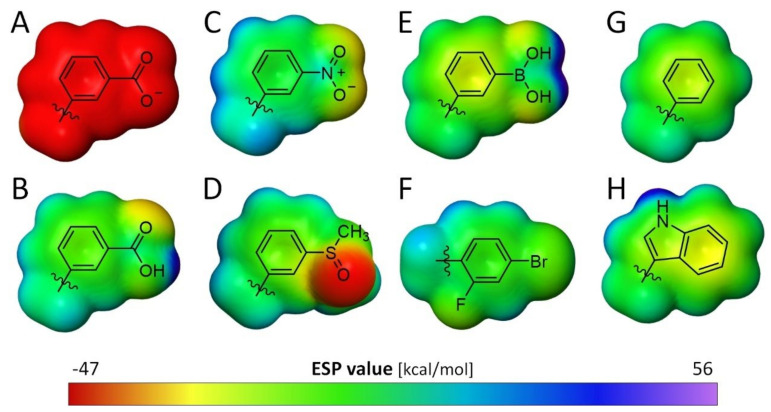
Figure 10.
Electronic surface potential area (ESP) of the terminal aromatic inhibitor moieties and an indole ring. (A): benzoate moiety, (B): benzoic acid moiety, (C): nitrophenyl moiety, (D): phenyl sulfoxide moiety, (E): phenylboronic acid, (F): 4-bromo-2-fluoro phenyl moiety, (G): phenyl moiety, and (H): indole moiety of a tryptophan calculated with the CC-PVTZ(-F)++ basis set and the M06-2X-D3 theory level, Jaguar, Schrödinger, LLC, New York, NY, USA, 2020 [23]. The graphical representation of the potential ranges from −47 kcal/mol (red) to +56 kcal/mol (purple).