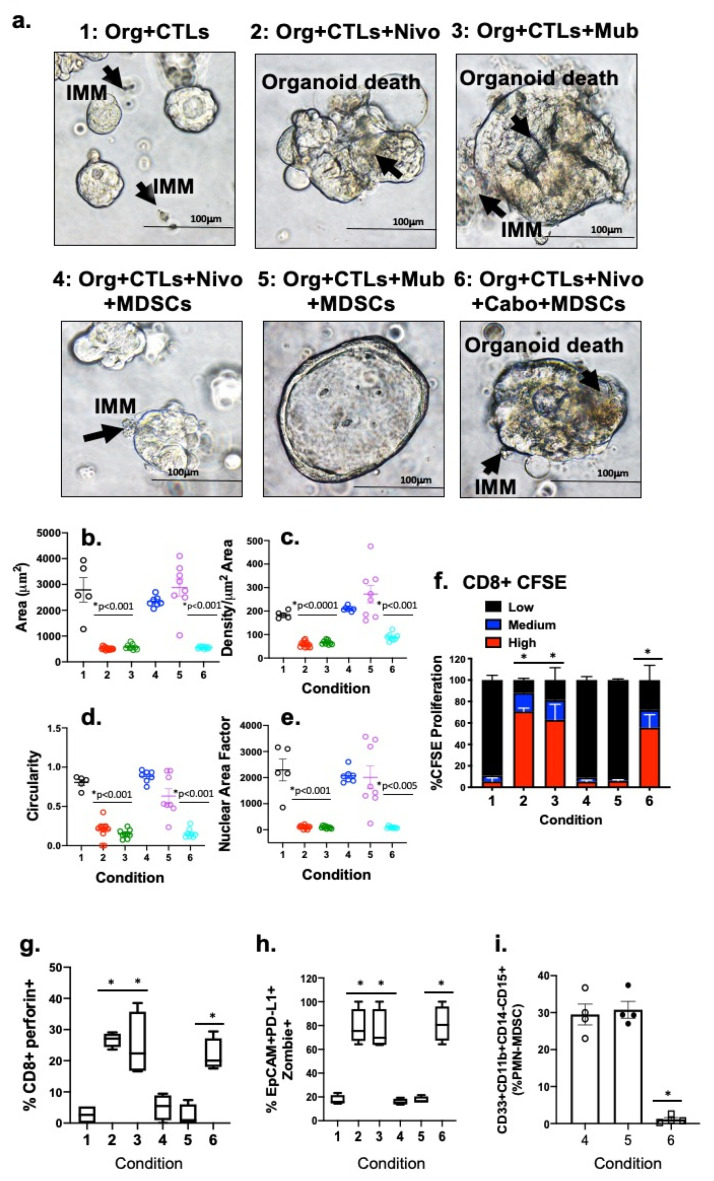
Figure 4.
Depletion of PMN-MDSCs sensitizes PD-L1 + huTGO/immune cell (IMM) co-cultures to Nivolumab. (a) Morphological alterations of huTGOs 48 h after the in vitro vehicle (Control) treatment in co-culture with CTLs (Condition 1), Nivolumab (0.5 µg/mL; Condition 2) or Mubritinib (6 nM; Condition 3); in co-cultures with CTLs, Nivolumab, and MDSCs (Condition 4) or CTLs, Mubritinib, and MDSCs (Condition 5); or Nivolumab and Cabozantinib together (10 µM, Condition 6) in co-cultures with CTLs and MDSCs. Quantitative changes in (b) area (c) density (d) circularity and (e) nuclear area factor. (f) Quantitative changes of CTL proliferation by CFSE, shown as percent (%) determined from flow cytometry data. Changes in (g) expression of perforin in CTLs, (h) percentage of viable EpCAM+/PD-L1+ huTGOs and (i) PMN-MDSCs after the treatments in Conditions 4 to 6. * p < 0.05 compared to Condition 1, shown here are n = 4 representative examples out of a total of 11 analyzed huTGO/immune cell co-cultures. Scale bar = 100 μm.