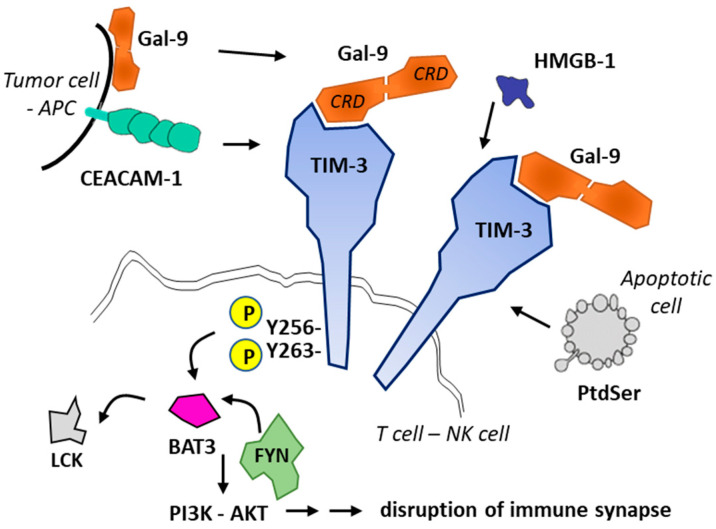
Figure 2.
Ligands and signaling of TIM-3. Four TIM-3 ligands have been identified: CEACAM1, phosphatidylserine (PtdSer released from apoptotic cells), HMGB-1 and Gal-9. Gal-9 can be secreted by antigen-presenting cells (APCs) or tumor cells or present at the cell surface. Gal-9 (with two carbohydrate recognition domains (CRD) separated by a short linker) promotes oligomerization of TIM3 and triggers signaling via phosphorylation of residues Tyr256 and Tyr263 in the intracellular domain of TIM3. The phosphorylation releases the adaptor protein BAT3 (HLA-B-associated transcript 3) and allows recruitment of tyrosine kinase FYN, whereas in its ligand-unbound form, TIM-3 interacts with BAT3 and recruits the kinase LCK to maintain T cell activation. Fyn and Bat3 are two adaptor molecules involved in inhibition and activation of Tim-3 downstream signaling, respectively. Gal-9-mediated recruitment of FYN leads to the disruption of immune synapse formation and to cell apoptosis [45].