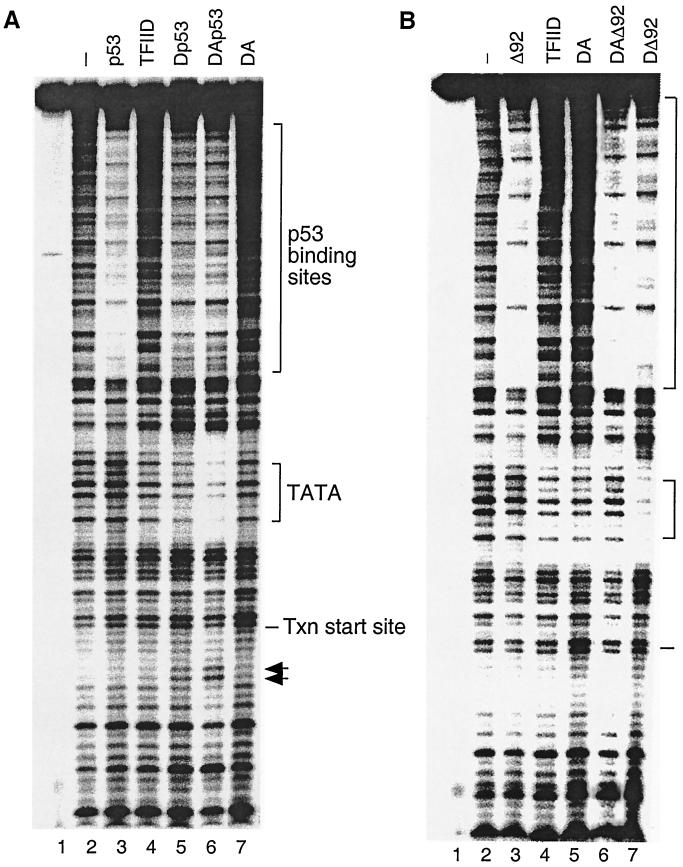
FIG. 2.
The conformational change of the DA complex is necessary for p53 transactivation. (A) p53, TFIIA, and TFIID cooperate to form a stable TFIID footprint on the promoter. In vitro DNase I footprint reactions were carried out as described in Materials and Methods. The amount of each factor added to the DNase I footprint reactions (as indicated above each lane) corresponds to 5 μl of TFIID, 100 ng of recombinant TFIIA, and 500 ng of p53. The positions of the p53 binding sites, the TATA box, and the transcription (Txn) start site are indicated on the right. Arrows denote hypersensitive sites. (B) ΔN92 (Δ92) fails to induce the conformational change of the DA complex on the promoter. The amount of each factor added to the DNase I footprint reactions (as indicated above each lane) corresponds to 5 μl of TFIID, 100 ng of recombinant TFIIA, 500 ng of p53, or 500 ng of ΔN92. The positions of the p53 binding sites, the TATA box, and the transcription start site are indicated on the right.