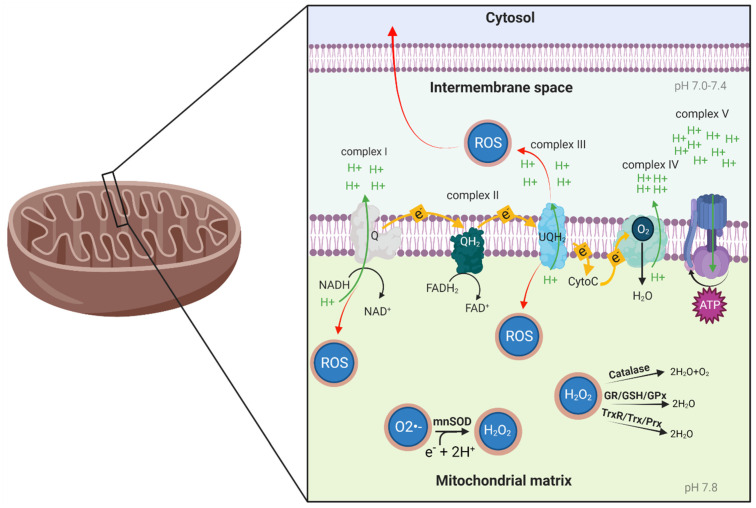
Figure 1.
Oxidative phosphorylation process and oxidative stress balance. NADH, a product of the citric acid cycle, is oxidized to NAD+ at complex I, transferring an electron to ubiquinone (Q). At complex II, FADH2 is oxidized to FAD+, transferring another electron to Q. When Q arrives at complex III, it transfers its electrons to cytochrome C (CytoC). At complex IV, the electrons carried by CytoC are drawn to diatomic oxygen (O2) and together with hydrogen, they form H2O. In the electron transport chain process, complexes I, III and IV pump protons from the mitochondrial matrix to the intermembrane space, creating an electrochemical proton gradient and a membrane potential (∆ψ) on the inner membrane of the mitochondria. Complex V (ATP synthase) uses the proton gradient to allow protons back into the mitochondrial matrix, synthesizing ATP in the process. Complexes I and III are the major sites of free radical production; if they release superoxide (O2•−) to the mitochondrial matrix, mnSOD will transform it to H2O2. Then, depending on cell specificity, H2O2 will be transformed to O2 and H2O by catalase, or to H2O by glutathione reductase/glutathione peroxidase (GR/GPx) or thioredoxin reductase/peroxiredoxin (TrxR/Prx) activity, in the presence of respiration products (i.e., NADPH).