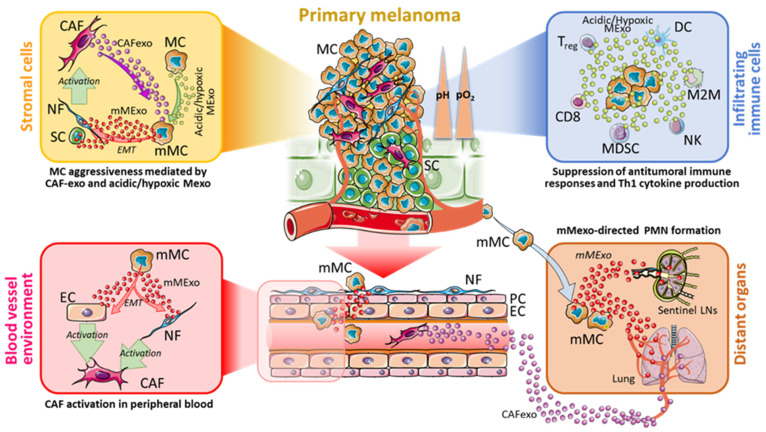
Figure 2.
Model of Exosome-based bi-directional crosstalk between CAFs, immune cells and Melanoma. During the development of the primary tumour, CAFs located in the TME release CAFexo which can increase the aggressiveness of MCs. In parallel, MCs in the acidic and hypoxic region (yellow box) can also release acidic and hypoxic Mexo, thus coordinating the generation of mMCs in the acidic and hypoxic regions of melanoma microenvironment, in part via EMT. These mMCs will produce mMexo which, through EMT, do elicit the activation of SCs and NFs into CAFs. In addition, mMCs will migrate into blood vessels and, by releasing mMexo, will contribute to the generation of PMN in distant organs (i.e., lung and LNs), which in turn become educated to receive the migrating mMCs (brown box). In the blood vessel environment (red box) mMCs and CAFs induce ECs and NFs to be activated into CAFs, in part by an EMT-dependent manner. CAFs will release CAFexo which participate in the PMN formation in distant organs, in coordination with mMexo. The acidic Mexo released by MCs will also reach the infiltrating immune cells (light blue box), leading to the activation of MDSCs, M2M and Tregs, and suppression of DCs and NKs. The final effect of such CAF-immune cells-melanoma crosstalk results in the generation of PMNs in distant organs, and the suppression of the anti-tumoural immune responses. CAFexo, CAF-derived exosomes; Mexo, melanoma-derived exosomes; mMexo, metastatic melanoma-derived exosomes; LNs, lymph nodes; NF, normal fibroblast; MC, melanoma cell; mMC, metastatic melanoma cell; EMT, Epithelial-to-mesenchymal transition; SC, skin cell; PC, pericyte; EC, endothelial cells; DC, dendritic cell; M2M, M2-differentiated macrophage; NK, natural killer cell, Treg, T regulatory lymphocyte; CD8, CD8+ T lymphocyte; MDSC, myeloid-derived suppressor cell; PMN, pre-metastatic niche.