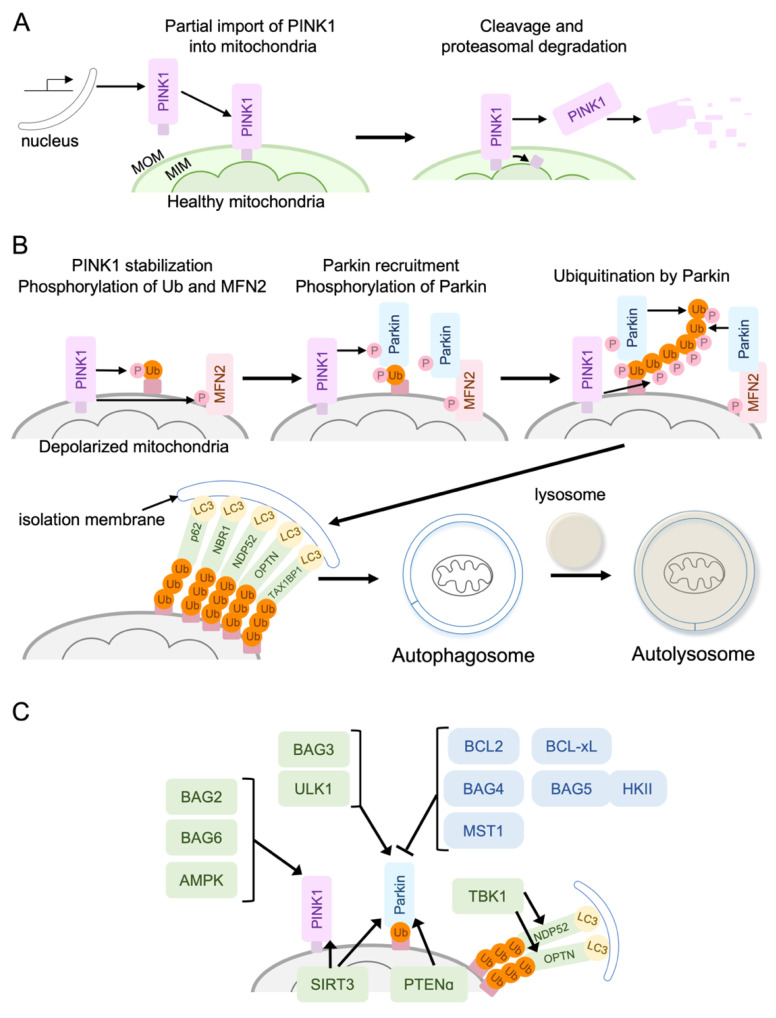
Figure 3.
The PINK1/Parkin-mediated mitophagy. (A) In healthy cells, PINK1, a mitochondrial serine/threonine kinase, is cleaved by an inner-mitochondrial membrane protease, and the cleaved PINK1 is then retro-translocated to the cytosol where it undergoes proteasomal degradation. (B) Under stress conditions, dissipation of mitochondrial membrane potential leads to the inhibition of PINK1 cleavage and supports the accumulation of full-length PINK1 at the MOM. PINK1 phosphorylates basal MOM ubiquitin which drives Parkin recruitment to the mitochondria and further phosphorylates Parkin to stabilize the binding of Parkin to ubiquitin. In addition, PINK1 phosphorylates mitofusin-2 (MFN2), a mitochondria fusion protein, which also serves as a receptor for Parkin at MOM. Ubiquitinated mitochondria are recognized by the autophagosome membrane through the autophagy receptor proteins, which interact with LC3 on the autophagosome membrane. (C) Signaling molecules regulating the PINK1/Parkin pathway.