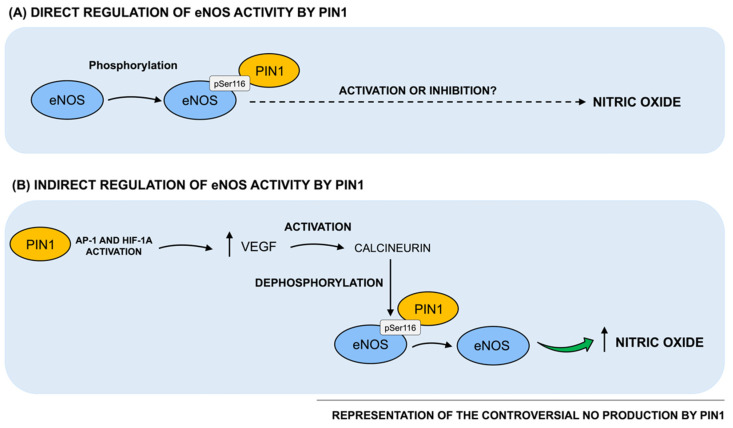
Figure 2.
Regulation of Pin1 direct and indirect effects on NO production. (A) Notably, Pin1 has been reported to interact with bovine eNOS in a phosphorylation-dependent manner. In particular, Pin1 binding site on bovine eNOS is at Ser116-Pro117, whose human equivalent is Ser114-Pro115. Such Pin1-catalyzed phosphorylation-dependent structural changes on eNOS have been reported to consequently impact on eNOS biological activity. However, based on data from the literature, two opposite series of results describing both a positive and a negative regulation of eNOS activity and, consequently, on NO production by Pin1, currently exist. (B) Pin1 has been found to indirectly regulate NO production by interacting with other key intracellular players, such as VEGF. Specifically, Pin1 overexpression has been shown to upregulate VEGF transcriptional activity and protein levels, by activating the transcription factor of AP-1 and HIF-1α. It has been hypothesized that Pin1 might increase VEGF expression that, in turn, stimulates the dephosphorylation and activation of eNOS at Ser116.