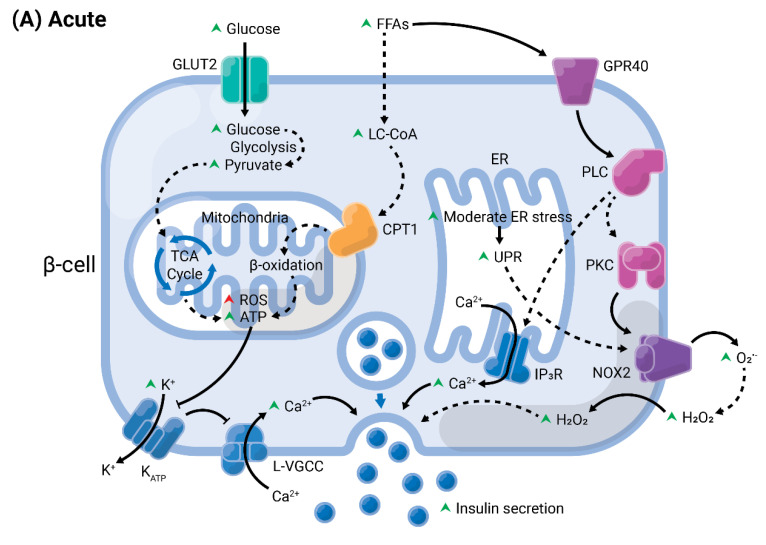
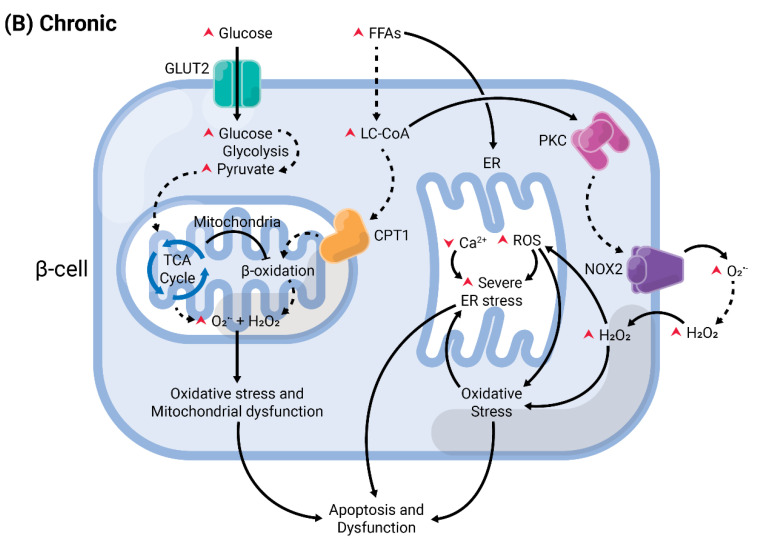
Figure 4.
Summary of acute versus chronic effects of fatty acids (FAs) in pancreatic β-cells. Glucose enters the β-cell through specific transporters located at the plasma membrane, GLUT2 (in rodents). After its phosphorylation by glucokinase, it undergoes various modifications by enzymes from the glycolysis, until the generation of pyruvate. This enters the mitochondria and is oxidized in the tricarboxylic acid (TCA) cycle. The electrons are transferred to the electron transport chain, resulting in the generation of reactive oxygen species (ROS), as byproducts, and ATP. (A) In acute conditions, FAs enter the cells and are converted into long-chain fatty-acyl CoA (LC-CoA), which is translocated to the mitochondria via carnitine palmitoyltransferase 1 (CPT1) to be oxidized in the β-oxidation, generating ATP and ROS. The increase of ATP/ADP leads to the closure of K+ channels sensitive to ATP (KATP) at the plasma membrane. The consequent membrane depolarization leads to the opening of L-type voltage-gated Ca2+ channels (L-VGCC). The rapid Ca2+ influx mobilizes insulin granules, which are released. Long-chain saturated or unsaturated FAs can also bind to Gαq-protein coupled receptor GPR40 at the plasma membrane. This activates the phospholipase C (PLC)/diacylglycerol (DAG) pathway, which respectively activates PKC and mobilizes Ca2+ from the endoplasmic reticulum (ER), potentiating GSIS. Activation of PKC may also activate NADPH oxidase 2 (NOX2) at the plasma to produce ROS, which are second messengers for GSIS. (B) Chronic exposure to FAs leads to depletion of ER Ca2+ and activation of ER stress, and potentiates ROS formation in all compartments (cytosol, mitochondria, and ER). Prolonged and unresolved oxidative stress, ER stress, and mitochondrial dysfunction culminate in apoptosis and dysfunction.